At what conditions do gases behave ideally?
Ideal gases are a theoretical concept, a simplified model of how gases behave. They are a good starting point for understanding real gases, but they don’t perfectly reflect reality.
So, at what conditions do gases behave ideally? Well, the answer lies in high temperatures and low pressures. Let’s explore why this is the case:
High temperatures mean that the gas molecules have a lot of kinetic energy, and they are moving around very quickly. This means they are less likely to interact with each other, and their behavior is closer to that of an ideal gas.
Low pressure means that the molecules are further apart, giving them more space to move around freely. This reduces the impact of intermolecular forces, which are forces that attract or repel gas molecules. In essence, at low pressure, the gas molecules are less likely to “bump into” each other.
Now, let’s get a bit more technical. The ideal gas law, which is a mathematical expression that describes the behavior of ideal gases, assumes that there are no intermolecular forces between gas molecules and that the molecules themselves have no volume. These assumptions, of course, are not entirely true for real gases. However, at high temperatures and low pressures, these assumptions become much closer to reality.
Think of it like this: imagine you’re in a crowded room, everyone is bumping into each other, and it’s hard to move around. That’s kind of like a gas at high pressure. Now imagine you’re in a spacious park with plenty of room to run around. That’s a lot like a gas at low pressure. The molecules have more space to move freely and are less likely to interact with each other.
As the temperature rises, the molecules have more energy and move faster, making them less likely to stick together. This means they spend less time in close proximity to each other, and intermolecular forces have less of an effect.
In summary, at high temperatures and low pressures, the behavior of real gases becomes more like the ideal gas model because the effect of intermolecular forces and the volume of the molecules become less significant.
When gas acts as ideal gas?
Let’s break it down. Higher temperatures and lower pressures are the key. Think of it this way: at higher temperatures, the gas molecules have more kinetic energy, which means they’re zipping around faster and bumping into each other more often. This makes the intermolecular forces between the molecules less significant compared to their kinetic energy.
At lower pressures, the molecules are further apart, so their size becomes less important compared to the empty space between them. This is why a gas behaves more ideally at higher temperatures and lower pressures.
Here’s a little more detail about why this happens:
Higher temperatures: When the temperature is high, the molecules have a lot of energy. They move around so fast that the attractive forces between them become almost negligible. Imagine a bunch of kids playing tag on a playground – they’re moving so fast that they barely notice each other.
Lower pressures: At lower pressures, the molecules are spread out further. This means there’s more space between them, and the attraction between them is even weaker. Think of the kids on the playground again – now imagine they’re playing on a huge field. They’re still moving fast, but now they have so much space that they barely interact with each other.
So, the ideal gas model works best when the molecules have enough energy to overcome the forces between them and when they’re far enough apart that their size doesn’t matter. This is why gases behave more ideally at higher temperatures and lower pressures.
Do gases always behave ideally?
Let’s break this down a bit. The ideal gas law is a simplified model that assumes gas particles have no volume and don’t interact with each other. In reality, gas particles do have volume, and they do interact (albeit weakly). These interactions become more significant at higher pressures and lower temperatures.
Think about it this way: at high pressure, gas molecules are squeezed together more tightly. This means they’re more likely to bump into each other and experience intermolecular forces. At low temperatures, gas molecules have less kinetic energy, making them more susceptible to these interactions. In these scenarios, the ideal gas law breaks down because the assumptions it’s based on aren’t valid.
On the other hand, at high temperatures and low pressures, gas molecules are farther apart and moving faster. This means they’re less likely to interact with each other. The behavior of these gases is closer to the ideal gas model.
In essence, the ideal gas law provides a useful framework for understanding gas behavior, especially under certain conditions. But, it’s important to remember that it’s a simplification, and real gases can deviate from this model, especially under extreme conditions.
Do gases behave most ideally at STP?
Let’s break down why low pressure and high temperature create ideal gas behavior:
Low pressure: Imagine a room filled with bouncing balls. If there are only a few balls, they have plenty of space to move around without bumping into each other. Similarly, at low pressure, gas molecules have more space to move freely, reducing the chances of collisions and interactions.
High temperature: Think of those bouncing balls again. If they’re moving slowly, they’re more likely to bump into each other and interact. But if you heat them up, they move faster and have less time to interact. In the same way, high temperatures give gas molecules more kinetic energy, allowing them to overcome attractive forces between molecules and behave more independently.
STP (Standard Temperature and Pressure) is defined as 0°C (273.15 K) and 1 atm pressure. While STP is a useful reference point, it’s important to remember that real gases don’t behave perfectly ideally at STP. The molecules still have some volume and experience some intermolecular forces. However, the deviation from ideal behavior is generally smaller at STP than at other conditions. Think of it like this: STP is a middle ground, not a perfect state of ideal gas behavior.
To sum it up, while gases at STP might show some ideal gas behavior, they behave more ideally at lower pressures and higher temperatures. This is because the molecules have more space to move around and have less interaction with each other.
How to know if a gas is behaving ideally?
At low pressures, gas particles are spread far apart. This means they don’t bump into each other very often, and the intermolecular forces between them are weak. Think of it like a group of people standing far apart in a huge field – they barely notice each other!
High temperatures give the gas particles more energy. This extra energy helps them overcome the intermolecular forces, letting them move around more freely. Imagine those people in the field now having a lot of energy and running around – they’re less likely to be affected by each other!
Now, let’s dive deeper into how ideal gas behavior comes into play. Ideal gases are theoretical, meaning they don’t perfectly exist in the real world. They’re basically a simplified model used to understand how gases behave. In this model, we assume that:
Gas particles have no volume. They’re just points with no size.
There are no intermolecular forces between gas particles. They act like they don’t even see each other!
So, when real gases are at low pressures or high temperatures, they behave more like the ideal gas model. This is because the intermolecular forces become less significant, and the particles act more like they have no volume.
This is why the ideal gas law works best in these conditions. It’s a simple equation that relates pressure, volume, temperature, and number of moles for a gas. The law is only an approximation for real gases, but it’s a pretty good one when those gases are at low pressures or high temperatures.
What gas conditions are ideal?
Imagine a classroom full of students. When there are only a few students, they can move around freely without bumping into each other. This is like a real gas at low pressure. However, when the classroom is crowded, the students start bumping into each other and it’s harder for them to move around. This is like a real gas at high pressure.
The same goes for temperature. At high temperatures, the students are more energetic and moving faster, so they have more freedom to move around. But at low temperatures, the students slow down and have less space to move around. This is similar to how the molecules in a real gas behave at different temperatures.
So, ideal gases are a great way to understand how gases behave, but they’re not a perfect representation of the real world. Real gases behave differently under different conditions, and it’s important to understand how these conditions affect their behavior.
What are the 5 assumptions of an ideal gas?
Here are the five core assumptions:
1. The particles in a gas are in constant, random motion. This means that gas molecules are always moving, bouncing around, and colliding with each other and the walls of their container. They don’t just sit still!
2. The combined volume of the particles is negligible. The molecules themselves are so small that their volume is insignificant compared to the overall volume of the container they occupy. It’s like the tiny specks of dust in a large room.
3. The particles exert no forces on one another. This means that the attractive or repulsive forces between gas molecules are negligible. They’re not really sticking to each other or pushing each other away. This assumption makes sense when the molecules are far apart, which is typical for gases.
4. Any collisions between the particles are completely elastic. In an elastic collision, kinetic energy is conserved. Think of a perfectly bouncy ball hitting a wall. No energy is lost during the collision. This implies that no heat is generated during the collision of gas molecules.
5. The average kinetic energy of the gas particles is proportional to the absolute temperature. This means that the faster the molecules move, the higher the temperature of the gas. The temperature is a direct measure of the average kinetic energy of the gas molecules.
Why are these assumptions important?
These five assumptions are critical because they allow us to develop a simple mathematical model that describes the behavior of ideal gases. This model, known as the ideal gas law, is a powerful tool for predicting and understanding the behavior of gases in a wide range of applications, from understanding how balloons inflate to predicting how engines work.
Now, real gases do deviate from these ideal behaviors, especially at high pressures or low temperatures when the molecules are closer together and intermolecular forces become more important. But for many applications, the ideal gas model provides a good approximation.
To summarize, the assumptions of an ideal gas are a simplification of reality, but they are incredibly useful for understanding and predicting the behavior of gases. By using these assumptions, we can make predictions about how gases behave in various conditions, which is essential in a wide variety of fields, from chemistry to engineering.
See more here: When Gas Acts As Ideal Gas? | When Do Gases Behave Ideally
Does a real gas exhibit ideal gas behavior?
These deviations arise from the fact that real gas molecules have finite size and interact with each other through attractive and repulsive forces. In contrast, ideal gas molecules are assumed to be point masses with no interactions. At high pressures, the molecules of a real gas are packed more closely together, leading to more significant intermolecular interactions and deviations from ideal behavior. At low temperatures, the molecules move more slowly, and the attractive forces between them become more pronounced, again leading to deviations from ideal behavior.
Let’s delve into this a bit deeper. Imagine a bunch of tiny billiard balls bouncing around in a room. These balls represent the molecules of an ideal gas – they have no size and don’t interact with each other. Now, imagine that the balls are replaced with real, slightly larger pool balls that can collide and interact. These pool balls represent real gas molecules.
The closer the pool balls get, the more they start bumping into each other and interacting. This bumping is like the intermolecular forces we talked about earlier. The more the pool balls bump and interact, the less they behave like the ideal billiard balls. Similarly, if the pool balls are moving very slowly, they’ll have more time to bump into each other and interact.
So, the key takeaway is that real gases don’t always behave perfectly like ideal gases. The closer they are to ideal behavior depends on how much they interact with each other, and this interaction is influenced by the pressure (how tightly they are packed) and the temperature (how fast they are moving).
What makes a gas an ideal gas?
An ideal gas is a theoretical concept that helps us understand how gases behave. In reality, no gas is truly ideal, but the concept is super useful for simplifying calculations and making predictions.
To be considered ideal, a gas needs to perfectly follow the gas laws at any temperature and pressure. This means the gas particles need to behave in a very specific way, according to the kinetic-molecular theory. Basically, the theory says that gas particles don’t take up any space themselves (zero volume) and they don’t attract each other at all.
Let’s break down those points a little more:
Zero volume: In the ideal gas world, we pretend that the gas particles are just tiny points with no size. This means that the particles don’t bump into each other and take up space, which is obviously not true in reality.
No attractive forces: In the ideal gas world, we imagine that the particles are completely independent of each other and don’t interact in any way. This means that there’s no pulling or pushing between the particles, which again isn’t exactly true in the real world.
So, why do we even talk about ideal gases if they don’t really exist? The answer is that they provide a great starting point for understanding real gases. By making these simplifying assumptions, we can develop equations that accurately predict how real gases will behave under many conditions.
For example, the ideal gas law (PV = nRT) is a very important equation that helps us understand the relationship between pressure, volume, temperature, and the number of moles of a gas. This equation is based on the concept of an ideal gas, but it works surprisingly well for many real gases, especially at low pressures and high temperatures.
Think of it this way: the ideal gas is like a simplified model that gives us a good starting point. We can then use this model to understand and predict the behavior of real gases, which are more complex and don’t always perfectly follow the rules.
Why does a gas behave more like an ideal gas?
Higher temperatures and lower pressures make a gas behave more like an ideal gas. This is because the kinetic energy of the gas molecules becomes more important than the potential energy due to intermolecular forces at higher temperatures. Think of it this way: the molecules are moving so fast that they barely notice each other, and their interactions are minimal.
At lower pressures, the molecules are farther apart, meaning the volume of the molecules is negligible compared to the empty space between them.
Here’s a deeper look at why these conditions lead to ideal gas behavior:
Intermolecular Forces: Real gases experience attractive forces between their molecules. These forces, like van der Waals forces, can cause the molecules to stick together slightly, affecting their movement and pressure. However, at high temperatures, the molecules have enough kinetic energy to overcome these attractions, making their behavior closer to that of an ideal gas where such forces are ignored.
Molecular Size: In a real gas, the molecules have a finite size, and this can influence their collisions and the volume they occupy. However, at low pressures, the molecules are spread out so far that their size becomes insignificant compared to the empty space between them. In this scenario, we can simplify our calculations by assuming the molecules have no volume, which is a defining characteristic of an ideal gas.
To summarize, when the kinetic energy of the molecules is much greater than the potential energy due to intermolecular forces, and when the molecular volume is insignificant compared to the volume of the container, the gas behaves more like an ideal gas.
Does a gas deviate from an ideal gas?
How Real Gases Differ from Ideal Gases
Another way to understand this is that cooling a gas will eventually turn it into a liquid. And a liquid definitely isn’t an ideal gas! You can see this in action with liquid nitrogen.
In a nutshell, real gases deviate most from ideal gas behavior at low temperatures and high pressures. Gases act most like ideal gases at high temperatures and low pressures.
Why Real Gases Act Differently at Low Temperatures and High Pressures
Let’s dive a little deeper into why real gases behave differently at low temperatures and high pressures.
Low Temperatures: At low temperatures, gas molecules slow down significantly. This means they have more time to interact with each other through attractive forces, which are ignored in the ideal gas model. These attractions cause the gas to deviate from ideal behavior.
High Pressures: At high pressures, gas molecules are squeezed together. This means they are much closer than they would be at lower pressures. These close distances lead to increased interactions between molecules, again causing deviations from ideal gas behavior.
Remember, the ideal gas model assumes no interactions between gas molecules and that they occupy negligible volume. In reality, gas molecules do interact with each other, and they do take up space. These factors are more pronounced at low temperatures and high pressures.
See more new information: bmxracingthailand.com
When Do Gases Behave Ideally? The Conditions And Why It Matters
You see, ideal gases are these theoretical entities that follow a set of rules. These rules are based on the idea that gas molecules are tiny, have no volume, and don’t interact with each other.
Now, in reality, no gas is *truly* ideal. All real gases have a bit of volume, and their molecules do interact, even if it’s just a little. So, when do gases act like their ideal counterparts? It’s all about the conditions.
High Temperature, Low Pressure: The Perfect Match for Ideal Behavior
The key to understanding when gases behave ideally is to understand the conditions that minimize the influence of those pesky real-world factors like volume and interactions.
High temperature plays a crucial role here. At high temperatures, the gas molecules are zipping around like crazy, their kinetic energy outweighing the weak intermolecular forces between them. Think of it like this: If you have a bunch of kids bouncing around a playground, they’re not going to spend much time clinging to each other. They’re too busy having fun and moving! Similarly, at high temperatures, the gas molecules are more focused on moving and less on interacting.
Low pressure, on the other hand, is also crucial. Low pressure means the molecules are far apart, minimizing their chances of bumping into each other and experiencing those pesky volume-related issues. Imagine those same kids on a huge playground. With lots of space to roam, they’re less likely to bump into each other and cause a ruckus.
The Ideal Gas Law: A Mathematical Framework for Ideality
The Ideal Gas Law, a fundamental equation in chemistry, describes the relationship between the pressure (P), volume (V), temperature (T), and number of moles (n) of an ideal gas:
PV = nRT
where R is the ideal gas constant.
This law is super useful for predicting the behavior of gases under different conditions. If we’re dealing with a real gas that’s behaving pretty ideally, we can use the Ideal Gas Law to make pretty accurate calculations.
Understanding Deviations from Ideality
Now, let’s talk about the real gases that don’t always behave ideally. When gases deviate from ideal behavior, it’s usually because:
Intermolecular forces (IMF) are stronger than we’d like. The molecules are hanging out together more than they should, affecting their movement and volume.
The gas molecules are relatively large. They take up a significant portion of the total volume, which throws off our calculations based on the assumption of negligible volume.
Common Examples
Let’s look at a few examples to illustrate when gases behave more or less ideally:
Helium (He) at room temperature and pressure. Helium is a tiny, lightweight gas with weak intermolecular forces. At room temperature and pressure, it behaves quite ideally.
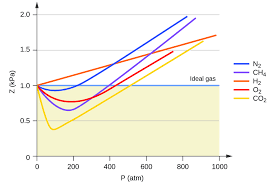
Nitrogen (N2) at high pressure. At high pressures, the nitrogen molecules are squeezed together, increasing their chances of interacting. This deviation from ideality becomes more pronounced at lower temperatures.
Water vapor (H2O) at low temperatures. Water vapor, especially at low temperatures, has strong hydrogen bonds, which lead to significant deviations from ideality.
So, How Ideal is Ideal?
It’s important to understand that no gas is *perfectly* ideal. Even the most ideal gas will show some deviations from ideality under certain conditions. But, for practical purposes, we can consider a gas to be “ideal” if its deviations are small enough to be negligible in our calculations.
Why Does it Matter?
So, why do we care about all this talk of ideal gases? Well, understanding the conditions under which gases behave ideally is crucial for many applications in chemistry and other sciences.
For example, in industrial processes involving gases, understanding how real gases behave under different conditions can help us:
Design and optimize chemical reactions. Knowing how gases behave helps us predict and control reaction rates and yields.
Develop efficient gas storage and transportation systems. We need to understand the compressibility of gases to design storage tanks and pipelines that can safely handle them.
Analyze and interpret experimental data. Understanding deviations from ideality is essential for accurately interpreting experimental data involving gases.
FAQs
Let’s answer a few common questions about ideal gases:
Q: What are some real-world examples of ideal gas behavior?
A: While no gas is perfectly ideal, gases like helium, hydrogen, and nitrogen at relatively high temperatures and low pressures often exhibit behavior close to ideal.
Q: How do I know if a gas is behaving ideally?
A: You can use the Ideal Gas Law to calculate the expected behavior of a gas under given conditions. If the experimental data matches the theoretical predictions, the gas is behaving ideally. If there are significant discrepancies, the gas is likely deviating from ideality.
Q: Are there any other factors that influence the ideality of gases?
A: Yes, factors like the molecular size and shape of the gas molecules can also influence their ideality. Larger and more complex molecules tend to exhibit greater deviations from ideality, as they have more opportunities to interact with each other.
Q: How can I learn more about ideal gases?
A: There are plenty of resources available to help you learn more about ideal gases. Check out your chemistry textbook, online resources like Khan Academy, and your local library.
Remember, the world of ideal gases is a fascinating one. By understanding the conditions under which gases behave ideally, we can unlock a deeper understanding of their behavior and use this knowledge to solve real-world problems in chemistry and beyond.
14.11: Real and Ideal Gases – Chemistry LibreTexts
Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases. Under what conditions then, do gases behave Chemistry LibreTexts
10.9: Real Gases – Deviations from Ideal Behavior
Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic assumptions behind the ideal gas Chemistry LibreTexts
Real Gas vs Ideal Gas – Science Notes and Projects
Learn the properties and differences between real gases and ideal gases, and when to use the ideal gas law or the van der Waals equation. Find out the factors that affect the behavior of real gases, Science Notes and Projects
15.4: Ideal Gases and The Ideal Gas Law – Chemistry LibreTexts
The volume (V) occupied by n moles of any gas has a pressure (P) at temperature (T) in Kelvin. The relationship for these variables, \[P V = n R T\] where R is Chemistry LibreTexts
Ideal Gas Law: Derivation, Assumptions, and the Dumas Method
The ideal gas law assumes that gases behave ideally, meaning they adhere to the following characteristics: (1) the collisions occurring between molecules are elastic and JoVE
Real and Ideal Gases – CK12-Foundation
Gases are most ideal at high temperature and low pressure. Nitrogen gas that has been cooled to 77 K has turned to a liquid and must be stored in a vacuum ck12.org
Non-ideal behavior of gases (article) | Khan Academy
The ideal gas equation is valid under all temperatures and pressures. So is the real gas equation. However, real gases behave ideally only in high temperatures and low pressures. Khan Academy
Ideal Gas Behavior – StatPearls – NCBI Bookshelf
Real gases behave ideally when subjected to either very low pressures or high temperatures. The low pressure of a system allows the gas particles to experience less intermolecular forces with National Center for Biotechnology Information
Real Gas And Ideal Gas
Real Gases: Deviations From Ideal Behavior | Ap Chemistry | Khan Academy
Kinetic Molecular Theory And The Ideal Gas Laws
The Ideal Gas Law: Crash Course Chemistry #12
Pressure In Gases | Matter | Physics | Fuseschool
When Do Real Gases Act Like Ideal Gases?
Real Vs Ideal Gases
Real Vs. Ideal Gases
Non-Ideal Gas Behavior
Mind-Blowing Theories On Nothingness You Need To Know | Documentary
Link to this article: when do gases behave ideally.
See more articles in the same category here: bmxracingthailand.com/what
