What is a singly ionized particle?
Imagine an atom like a tiny solar system, with a nucleus at the center and electrons orbiting around it. When an atom is singly ionized, one of these electrons is kicked out of its orbit, leaving the atom with one less negative charge. This imbalance in charge is what creates the positive charge.
Singly ionized particles are common in many situations, from the natural world to the lab. For example, in the upper atmosphere, ultraviolet radiation from the sun can knock electrons out of atoms, creating a layer of singly ionized particles known as the ionosphere. This layer plays a crucial role in reflecting radio waves, allowing us to communicate over long distances.
In laboratories, singly ionized particles are often created using methods like electron impact ionization, where a beam of electrons is used to knock electrons out of atoms. These singly ionized particles are then used in a variety of applications, such as mass spectrometry, which helps us identify different molecules based on their mass-to-charge ratio.
What is a singly ionized helium atom?
The ionization energy is the amount of energy needed to completely remove this remaining electron. Since the electron is in the first orbit, the ionization energy is also equal to the kinetic energy of that electron in the first orbit.
It’s kind of like a mini-solar system! The nucleus (the center of the atom) acts like the sun, and the electron zips around it like a planet. The farther away the electron is from the nucleus, the less energy it takes to remove it.
But in the case of a singly ionized helium atom, the electron is as close as it can get to the nucleus, so it takes a lot of energy to pull it away. This makes singly ionized helium atoms very stable and useful in applications like lasers and spectroscopy.
What is singly ionized carbon?
Let’s dive into the fascinating world of singly ionized carbon! It’s essentially a carbon atom that has lost one of its electrons, making it positively charged. Think of it as a carbon atom with a slight “missing piece.”
Now, you might be wondering how this happens. Well, a photon with an energy of 11.3 eV can ionize a carbon atom. This means the photon’s energy is enough to knock off one electron from the carbon atom, leaving it with a positive charge. This process is called photoionization.
Once a carbon atom is singly ionized, it becomes C+. This C+ ion has a special trick up its sleeve – it can emit a fine-structure line at a specific wavelength, 157.7 μm, when it’s in the right environment.
This fine-structure line is a kind of “fingerprint” that lets us know C+ is present. Imagine it like a specific sound that tells us a particular instrument is playing.
To see this line, we need a special condition: a critical density of about 3 x 103 cm-3. Think of this density as the number of C+ ions per cubic centimeter. This is important because it tells us how many C+ ions are packed together in a particular region, and this affects their behavior.
Why is singly ionized carbon so interesting?
Singly ionized carbon (C+) is an important player in many astronomical and astrophysical environments. It’s particularly interesting for understanding things like:
Interstellar clouds: These clouds are vast regions of gas and dust that are the birthplace of stars. C+ is often found in these clouds and can help us understand their composition and temperature.
Planetary nebulae: These are colorful shells of gas ejected from dying stars. C+ plays a role in the dynamics of these nebulae and its emission helps us study their evolution.
The early universe: C+ was one of the first ions to form after the Big Bang and is a key indicator of the early universe’s conditions.
Singly ionized carbon is like a detective, helping us unravel the mysteries of the universe. By studying its behavior and emission, we can gain valuable insights into the composition, temperature, and evolution of various celestial objects.
What does ionizing mean?
Ionization is the process of turning an electrically neutral atom or molecule into a charged atom or molecule, called an ion. This happens when an atom either gains or loses electrons, the tiny particles that carry a negative charge.
Think of it like this: Imagine a neutral atom as a perfectly balanced scale, with the same number of positive and negative charges. When an atom ionizes, it’s like adding or removing weights from one side of the scale. If the atom gains an electron, it becomes negatively charged because it has more negative charges than positive ones. If it loses an electron, it becomes positively charged because it has more positive charges than negative ones.
Ionization is a fundamental process in chemistry and physics, and it plays a crucial role in many important phenomena, including:
Chemical reactions: Ionization can lead to the formation of new chemical bonds, driving chemical reactions.
Electrical conductivity: Ions can conduct electricity, making solutions and materials conductive.
Spectroscopy: The interaction of light with ions can be used to identify and analyze different substances.
Plasma formation: Ionization is the process that creates plasma, the fourth state of matter.
Understanding ionization is essential for understanding how atoms and molecules behave in different environments.
What does singly ionised mean?
To understand this, think about atoms as being like tiny building blocks of matter. Atoms are made up of even tinier particles: protons, neutrons, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. Normally, an atom has an equal number of protons and electrons, which means its overall charge is neutral.
But when an atom loses an electron, the balance is disrupted. It now has more protons than electrons, leading to a net positive charge. This process is called ionization, and the atom is now considered a singly ionized ion.
For example, a hydrogen atom (H) has one proton and one electron. When it loses its electron, it becomes a singly ionized hydrogen ion (H+), which is simply a proton. This singly ionized hydrogen ion is extremely important in many chemical reactions and plays a critical role in the universe.
The loss of an electron can happen in various ways. One common way is through interactions with other atoms or molecules. For instance, when a hydrogen atom bumps into another atom, the energy from the collision might be enough to knock off its electron, resulting in singly ionization.
It’s important to remember that singly ionized is just one type of ionization. Atoms can lose multiple electrons, becoming doubly, triply, or even higher-order ionized ions. The number of lost electrons determines the ion’s charge.
What does fully ionized mean?
Think of it this way: Imagine you have a bunch of atoms, and they’re all happily holding onto their electrons. But then you heat things up, really really hot. The electrons get so excited they literally fly off the atoms, leaving behind just the nucleus. That’s when you have a fully ionized atom.
This is what happens in really hot places like the Sun and stars. The intense heat creates a special type of gas called plasma where the atoms are fully ionized. This kind of plasma is a hotbed of energy and plays a vital role in how stars generate light and heat. It’s also the stuff of nuclear explosions, where the extreme temperatures create even hotter plasmas.
What does it mean to be fully ionized?
When an atom is fully ionized, it essentially becomes a positively charged particle, because it’s missing all those negatively charged electrons. This is important because it changes how the atom interacts with other atoms and its environment. For instance, since a fully ionized atom has no electrons, it can’t form chemical bonds. That means it doesn’t behave like a regular atom in a chemical reaction.
Think of it as a single piece of Lego that’s lost all its connecting studs. It can’t connect to other Legos and build anything new. The same goes for a fully ionized atom. It’s lost its ability to participate in the intricate dance of chemical reactions.
Instead, it takes on a life of its own, interacting with other charged particles through the force of electricity. That’s why fully ionized atoms are found in plasmas, which are essentially a “soup” of charged particles.
Fully ionized atoms play a critical role in many areas of physics and astrophysics. They are responsible for the light and heat of stars, the power of nuclear explosions, and the behavior of many other cosmic phenomena.
Are singly ionized atoms accelerated?
Imagine you have an atom that has lost one electron. We call this a singly ionized atom. These atoms are positively charged because they now have more protons than electrons. Now, if we put these positively charged atoms in an electric field, they’ll feel a force that pushes them towards the negatively charged side of the field. This is because opposite charges attract. This force causes the ions to accelerate, gaining speed as they move.
Here’s how it works in a velocity selector:
Singly ionized atoms (one electron removed) are accelerated and then passed through a velocity selector consisting of perpendicular electric and magnetic fields. The electric field is 155 V/m, and the magnetic field is 0.0315 T.
The velocity selector is a special device that lets only ions with a specific velocity pass through it. It uses both electric and magnetic fields to achieve this. The electric field pushes the ions in one direction, while the magnetic field pushes them in a perpendicular direction. Only ions traveling at a specific velocity will experience forces that perfectly balance each other out, allowing them to pass straight through the selector.
Let’s delve a little deeper into how this works.
The electric field exerts a force on the ions, causing them to accelerate. The magnetic field, on the other hand, exerts a force on the moving ions, perpendicular to their motion. The strength of the magnetic force depends on the speed of the ion.
Think of it this way: If the ion is moving too slow, the magnetic force won’t be strong enough to counteract the electric force, and the ion will be deflected. If the ion is moving too fast, the magnetic force will be too strong, and the ion will also be deflected.
Only ions moving at a specific velocity will experience a balance between the electric and magnetic forces, allowing them to pass through the velocity selector undisturbed. This specific velocity is determined by the strength of the electric and magnetic fields.
The velocity selector is a crucial tool in many scientific experiments and applications. It allows researchers to isolate ions with a specific velocity, making it possible to study their properties or use them in other processes.
See more here: What Is A Singly Ionized Helium Atom? | What Does Singly Ionized Mean
What is ionization in chemistry?
Ionization is a fascinating process in chemistry that involves the formation of ions, which are atoms or molecules carrying a positive or negative charge. This happens when an atom or molecule gains or loses electrons, often in conjunction with other chemical changes.
Think of it like this: Imagine an atom as a neutral object. When it gains an electron, it becomes negatively charged, forming an anion. Conversely, when it loses an electron, it becomes positively charged, forming a cation. This change in charge is what defines an ion.
Here’s why ionization is important:
It’s the basis of many chemical reactions: Ionization plays a crucial role in reactions like acid-base reactions, where protons (H+) are transferred. It also fuels redox reactions involving the transfer of electrons.
It’s essential for the formation of compounds: Many compounds, like salts, are formed when cations and anions attract each other due to their opposite charges.
It’s key to understanding the behavior of matter: Ionization can influence the physical properties of a substance, like its conductivity, melting point, and boiling point.
Let’s delve a bit deeper into the process of ionization:
Ionization Energy: You might be wondering how much energy is needed to remove an electron from an atom. This is where ionization energy comes in. It’s the minimum amount of energy required to remove an electron from a gaseous atom in its ground state. The ionization energy is usually measured in electron volts (eV) or kilojoules per mole (kJ/mol).
Factors Affecting Ionization Energy:
Several factors can influence the ionization energy of an atom:
Nuclear Charge: The higher the nuclear charge (the number of protons), the stronger the attraction between the nucleus and the electrons, making it harder to remove an electron and thus increasing the ionization energy.
Electron Shielding: The core electrons can shield the outer electrons from the nucleus’s full attraction. This shielding reduces the ionization energy.
Atomic Radius: Smaller atoms have a higher ionization energy because their electrons are closer to the nucleus, experiencing a stronger attraction.
In conclusion, ionization is a fundamental process in chemistry that explains the formation of ions and their involvement in various chemical reactions and the properties of matter. Understanding ionization energy and its influencing factors helps us gain insight into the behavior of atoms and molecules.
What happens if most atoms are ionized?
While plasma is electrically neutral on a large scale, meaning it has an equal number of positive and negative charges, it’s a dynamic environment where the ions and electrons interact constantly. This constant movement and interaction give plasma unique properties, making it quite different from regular gases.
Think of it like this: A regular gas is like a bunch of marbles bouncing around in a box. In plasma, those marbles are charged, and they’re constantly jostling each other, creating a chaotic yet fascinating dance of charged particles. This constant interaction gives plasma the ability to conduct electricity and respond strongly to magnetic fields.
Plasma is more common than you might think. It’s found in stars, lightning, fluorescent lights, and even the aurora borealis. It’s also being studied for its potential applications in areas like energy production, medicine, and space exploration.
So, while a world where most atoms are ionized might sound like something out of a science fiction movie, it’s a very real and fascinating state of matter that plays a significant role in the universe.
What causes ionization?
There are a few ways an atom can ionize. It can happen when an atom collides with subatomic particles, other atoms, molecules, electrons, positrons, protons, antiprotons, or ions. It can also happen when an atom interacts with electromagnetic radiation.
Let’s break these down a bit more:
Collisions: When an atom collides with another particle, like an electron or a photon, it can transfer energy. If enough energy is transferred, the atom can lose an electron, becoming a cation. This is a common process in plasmas, which are gases that are ionized.
Electromagnetic Radiation:Electromagnetic radiation, such as light, can also cause ionization. When an atom absorbs a photon with enough energy, an electron can be ejected from the atom. This process is called the photoelectric effect. The amount of energy needed to ionize an atom depends on the element in question and its electron configuration.
Ionization is a fundamental process in chemistry and physics. It plays a vital role in many natural phenomena, such as lightning, auroras, and the formation of stars. It also has many applications in technology, such as in lighting, lasers, and semiconductor manufacturing.
How does ionization work?
Think of it like a game of pool, but with atoms! The speeding electrons are like the cue ball, and the gas molecules are the other balls. When the cue ball hits another ball with enough force, it knocks it loose. In ionization, the cue ball (electron) has to have enough energy to overcome the attraction between the electron and the atom. This amount of energy is called the ionization energy, and it’s different for each type of atom.
Here’s a cool thing: the freed electrons can go on to knock more electrons loose from other molecules, creating a chain reaction. This is how ionization happens. It’s a process that creates charged particles from neutral particles, and it’s crucial for many things, like making lightning and fluorescent lights work.
See more new information: bmxracingthailand.com
What Does Singly Ionized Mean: A Simple Explanation
In a nutshell, “singly ionized” means that an atom has lost one electron.
Let’s break it down:
Atoms: Remember those tiny building blocks of everything? They have a nucleus in the center, with protons (positively charged particles) and neutrons (no charge). Orbiting this nucleus are electrons, which carry a negative charge.
Ions: Now, atoms are usually neutral, meaning they have an equal number of protons and electrons. But sometimes, they can gain or lose electrons. When this happens, they become ions.
Singly Ionized: If an atom loses one electron, it becomes positively charged. We call this singly ionized. Think of it like this: The atom has one more proton (positive) than electrons (negative), so it’s got a net positive charge.
An Example to Help
Let’s imagine a sodium atom (Na). It has 11 protons and 11 electrons. If it loses one electron, it becomes Na+, meaning it’s now singly ionized.
Why Does This Happen?
Atoms don’t just randomly lose electrons. It often happens during chemical reactions or when they’re exposed to energy sources like heat or light.
A Few Points to Remember
Charge: A singly ionized atom has a +1 charge.
Notation: We use a superscript + sign to represent a singly ionized atom (like Na+).
Reactivity: Ionization changes an atom’s reactivity. Singly ionized atoms can behave differently in chemical reactions compared to their neutral counterparts.
Beyond Singly Ionized
You might also hear about doubly ionized, triply ionized, or even highly ionized atoms. These simply mean the atom has lost two, three, or even more electrons, respectively.
FAQs About Singly Ionized
1. What is the difference between a singly ionized atom and a neutral atom?
Neutral atom: Equal number of protons and electrons. No net charge.
Singly ionized atom: Lost one electron. Net charge of +1.
2. Can an atom gain an electron instead of losing one?
Yes! If an atom gains an electron, it becomes negatively charged and is called an anion. For example, if a chlorine atom (Cl) gains an electron, it becomes Cl- (singly ionized anion).
3. How can I tell if an atom is singly ionized?
Look for the superscript + sign after the element symbol. For example, Na+ means sodium is singly ionized.
4. Where do I encounter singly ionized atoms in everyday life?
Singly ionized atoms are everywhere! They play a role in:
Electrochemistry: Batteries, for instance, rely on the movement of ions, including singly ionized ones.
Lighting: Fluorescent bulbs and neon signs use ionized gases to produce light.
Plasma: The fourth state of matter (after solid, liquid, and gas). Plasma is a collection of ionized atoms and electrons.
5. Is singly ionized the same as positively charged?
Yes, for an atom, being singly ionized *means* it’s positively charged.
6. What are some examples of singly ionized atoms?
* Sodium ion (Na+)
* Potassium ion (K+)
* Calcium ion (Ca2+) – This one has lost two electrons, making it doubly ionized.
7. What are some real-world applications of singly ionized atoms?
Mass spectrometry: Used to identify and analyze molecules by separating ions based on their mass-to-charge ratio.
Medical imaging: Positron Emission Tomography (PET) scans use radioactive isotopes, which often decay to form singly ionized atoms.
Semiconductors: Used in transistors and other electronic devices to control the flow of electrons.
Let me know if you have any other questions! I’m always here to help you understand the fascinating world of atoms and ions.
Ionization | Definition, Examples, & Facts | Britannica
ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted to electrically charged atoms or molecules ( ions) Britannica
What Does it Mean to be Singly Ionized? – Spring Constant of DNA
What is the definition of being singly ionized? Singly ionized refers to an atom or molecule that has lost one electron, resulting in a net positive charge. What are Physics Forums
Ionization and Plasmas – University of Rochester
If most of the atoms or molecules in a region are ionized, the resulting state of matter corresponds to a gas that is electrically neutral on a global scale, but composed rochester.edu
6.5: Bohr’s Model of the Hydrogen Atom – Physics LibreTexts
When one of the orbiting electrons is removed from the helium atom (we say, when the helium atom is singly ionized), what remains is a hydrogen-like atomic Physics LibreTexts
Ionization: Definition, Process, and Examples | Electrical4U
Ionization occurs when an atom or molecule gains or loses one or more electrons, resulting in a positive or negative charge. The charged atom or molecule is Electrical4U
CH104: Chapter 3 – Ions and Ionic Compounds – Chemistry
Mercury is unusual in that its singly ionized oxidation state, mercury(I), is found as a dimeric cation, Hg 2 2+, where two atoms of mercury are actually covalently bonded to one another as a polyatomic ion. Each Western Oregon University
Ionization in Spectra – umop.net
So when energized, most atoms will typically be neutral atoms, but some will be singly ionized, a few will be doubly ionized, even fewer yet will be triply ionized, and so on. umop.net
Ionization Energies – Chemistry LibreTexts
All elements have a first ionization energy – even atoms which do not form positive ions in test tubes. The reason that helium (1st I.E. = 2370 kJ mol-1) does not normally form a positive ion is because of Chemistry LibreTexts
Example Singly Ionized Helium
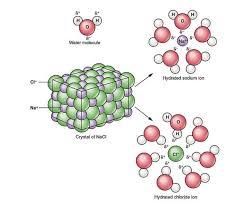
What Is Ionization? Example Of The Ionisation Process Using Sodium Chloride (Nacl) | Electrical4U
A Singly Ionized Particles Experiences
What Is The Wavelength Of The 2 Αtransition In Singly Ionized Helium? (Hint: The Difference B…
Atomic Spectra Of Singly Ionized Helium Atom
In The Spectrum Of Singly Ionized Helium, The Wavelength Of A Line Observed Is Almost The Same As…
Hydrogen Ion And Singly Ionized Helium Atom Are P Accelerated, From…
Warning: Do Not Try—Seeing How Close I Can Get To A Drop Of Neutrons
Gömböc—The Shape That Shouldn’T Exist
Pure Information Gives Off Heat
Link to this article: what does singly ionized mean.
See more articles in the same category here: bmxracingthailand.com/what
