What kind of fatty acid has the lowest melting point?
Let’s break down why this is the case. The melting point of a fatty acid is influenced by its structure, particularly the number of double bonds present. Double bonds create kinks in the fatty acid chain, making it more difficult for the molecules to pack together tightly. This looser packing results in weaker intermolecular forces, leading to a lower melting point.
Linoleic acid is a polyunsaturated fatty acid, meaning it has multiple double bonds. In fact, it has two double bonds, which significantly disrupt the linear structure of the fatty acid chain. This increased number of double bonds, compared to other essential fatty acids like alpha-linolenic acid (which has three double bonds) or oleic acid (which has one double bond), contributes to its lower melting point.
Think of it like this: imagine trying to stack a bunch of bendy straws versus straight straws. The bendy straws (like linoleic acid with its double bonds) will be much harder to stack neatly and tightly compared to the straight straws. This makes linoleic acid a liquid at room temperature, while other fatty acids with fewer double bonds might be solid.
Which types of fats have lower melting points?
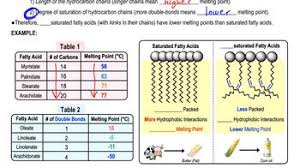
Unsaturated fatty acids generally have lower melting points than saturated fatty acids and are often liquid at room temperature. This is because the structure of their molecules is different.
Saturated fatty acids have a straight chain structure, allowing them to pack tightly together. This tight packing makes them more solid at room temperature. Think of it like neatly stacked logs – they form a solid structure.
Unsaturated fatty acids, on the other hand, have one or more double bonds in their chain. These double bonds create kinks in the molecule, preventing them from packing as tightly. Imagine trying to stack logs with a bend in them – it’s harder to make a solid structure.
Here are a few examples:
Saturated fatty acids: Behenic acid, stearic acid, and palmitic acid.
Unsaturated fatty acids: Oleic acid, linoleic acid, and linolenic acid.
Let’s dive deeper into the why behind this difference in melting points:
The double bonds in unsaturated fatty acids influence their melting point by affecting the intermolecular forces between the molecules. Intermolecular forces are the attractive forces that hold molecules together.
Saturated fatty acids, with their straight chains, can pack tightly and have strong intermolecular forces, which require a lot of energy to overcome – hence, a higher melting point.
Unsaturated fatty acids, with their kinks, have weaker intermolecular forces because they can’t pack as tightly. This means less energy is needed to break these weaker forces, leading to a lower melting point.
Think of it like this: imagine you have a bunch of magnets. The stronger the magnets, the harder it is to pull them apart. Saturated fatty acids are like strong magnets, while unsaturated fatty acids are like weaker magnets. The weaker magnets need less force to break them apart, just like the unsaturated fatty acids need less energy to melt.
So, the next time you’re thinking about fats, remember that the presence of double bonds in unsaturated fatty acids is the key to their lower melting point and liquid state at room temperature!
Which fatty acid has the highest melting point?
Why? Well, it’s all about the length of the fatty acid chain. CH3(CH2)14COOH has the longest chain out of the bunch. And the longer the chain, the higher the melting point.
Think of it this way: longer chains have more surface area, which allows for stronger intermolecular forces (like Van der Waals forces) between the molecules. Stronger forces mean it takes more energy (heat) to break those bonds and melt the fatty acid.
Here’s the deal: The melting point of a fatty acid isn’t just about the chain length. The level of saturation also plays a big role. Saturated fatty acids have all single bonds between their carbon atoms. Think of it like a straight, tightly packed chain. These chains pack together neatly, leading to a higher melting point. On the other hand, unsaturated fatty acids have at least one double or triple bond, which creates a “kink” in the chain. These kinks prevent the molecules from packing as tightly, making them melt at a lower temperature.
Let’s break down the relationship between fatty acid chain length, saturation, and melting point.
Chain Length
Longer chains: More surface area for intermolecular forces. This means stronger bonds and a higher melting point.
Shorter chains: Less surface area for intermolecular forces. This means weaker bonds and a lower melting point.
Saturation
Saturated fatty acids: No double or triple bonds. Straight chains pack tightly, leading to a higher melting point.
Unsaturated fatty acids: At least one double or triple bond. The “kinks” in the chain prevent tight packing, leading to a lower melting point.
Think of it like this: Saturated fatty acids are like neatly stacked logs in a woodpile. They’re all packed tightly, and it takes a lot of force to separate them. Unsaturated fatty acids are like those logs, but with some of them cut at an angle. They don’t pack as tightly, and it’s easier to separate them.
So, CH3(CH2)14COOH is the champion because it has the longest chain, and it’s saturated, meaning it has no kinks to interrupt its tight packing.
Why do cis fatty acids have a lower melting point?
You see, cis fatty acids have a kinked shape, which makes it harder for them to pack together tightly. Think of it like trying to stack a bunch of bent straws – it’s just not as efficient as stacking straight straws. This looser packing means that cis fatty acids have weaker intermolecular forces, leading to a lower melting point.
To illustrate this further, imagine you’re trying to build a tower out of blocks. If the blocks are perfectly rectangular, they stack neatly and make a strong, stable tower. But if some of the blocks are bent, it becomes much harder to build a tall, sturdy tower. It’s the same with fatty acids – the kinks in cis fatty acids make it difficult for them to pack tightly, leading to a weaker structure and a lower melting point.
Trans fatty acids, on the other hand, have a straighter shape, similar to saturated fatty acids. This allows them to pack together more tightly, resulting in stronger intermolecular forces and higher melting points.
It’s important to remember that cis fatty acids are naturally occurring in many foods, especially those derived from plants. They are often considered healthier than trans fatty acids, which are primarily created through industrial processes. So, while cis fatty acids may have a lower melting point, they play an important role in our diet and overall health.
Which of the following fatty acid has lower melting point?
The presence of double bonds in a fatty acid chain affects its melting point. Saturated fatty acids, which have no double bonds, are more tightly packed together, leading to stronger intermolecular forces and a higher melting point. In contrast, unsaturated fatty acids, which have one or more double bonds, have “kinks” in their chains. These kinks prevent the molecules from packing closely together, resulting in weaker intermolecular forces and a lower melting point. The more double bonds a fatty acid has, the lower its melting point will be.
For example, oleic acid has one double bond and melts at 13.4°C, while linoleic acid has two double bonds and melts at -5°C. This difference in melting point is significant, as it affects the physical properties of fats and oils. Fats with a higher melting point are solid at room temperature, while those with a lower melting point are liquid. This is why butter, which is primarily composed of saturated fatty acids, is solid at room temperature, while olive oil, which is rich in oleic acid, is liquid.
Which has the lowest melting point list?
Let’s dive a little deeper into why Mercury is so unique.
It’s all about the atomic structure and how the atoms are bonded together. Mercury’s atoms have a special arrangement that makes it really easy for them to move around, even at low temperatures. This is because the electrons in Mercury’s outer shell are very weakly bound to the atom, meaning they can easily be shared with other atoms. This “loose” arrangement makes Mercury a good conductor of electricity and heat, and it also makes it liquid at room temperature.
Now, imagine a metal like iron. It has a much higher melting point because its atoms are tightly bound together. Iron atoms are much larger than Mercury atoms, and they have more electrons in their outer shell. This means the electrons are more strongly bound to the atom, making it harder for them to share with other atoms. So, it takes a lot more energy to break those bonds and make iron melt.
It’s fascinating how the arrangement of atoms can have such a big impact on the properties of a material! Think about it—if Mercury had a higher melting point, it wouldn’t be used in thermometers, and many other applications would be different. It’s a good thing that this unique element melts at such a low temperature!
See more here: Which Types Of Fats Have Lower Melting Points? | Which Fatty Acid Has The Lowest Melting Point
Which fatty acid has the highest melting point?
We know that the number of carbon atoms in a fatty acid directly impacts its melting point. The more carbons, the higher the melting point.
Now, let’s look at our fatty acids:
1. CH3 (CH2)14COOH – This fatty acid has the most carbons (16 total) and therefore will have the highest melting point.
2. CH3 (CH2)12COOH – This fatty acid has 14 carbons.
3. CH3 (CH2)6 (CH=CHCH2)2 (CH2)COOH – This one has 18 carbons, but the double bonds introduced by the (CH=CHCH2)2 group make it unsaturated. Unsaturated fatty acids have lower melting points than their saturated counterparts with the same number of carbons.
4. CH3 (CH2)2COOH – This fatty acid has 4 carbons.
So, our final arrangement from highest melting point to lowest would be:
1. CH3 (CH2)14COOH
2. CH3 (CH2)12COOH
3. CH3 (CH2)6 (CH=CHCH2)2 (CH2)COOH
4. CH3 (CH2)2COOH
Why does the number of carbons matter?
Think of it like this: carbon chains are attracted to each other through van der Waals forces. These forces get stronger as the chains get longer (more carbons). Longer chains can interact more effectively, leading to a more stable, solid state – and a higher melting point!
But what about those double bonds?
Double bonds create kinks in the fatty acid chain. These kinks disrupt the close packing of the molecules, making it harder for them to solidify. Imagine trying to stack a bunch of floppy straws versus stiff pencils – the straws are more likely to fall over! That’s why unsaturated fatty acids have lower melting points.
What is the melting point of a pure fatty acid?
Let’s break it down. DSC is a powerful tool used to analyze how materials respond to changes in temperature. It’s like a mini-oven that carefully heats up a tiny sample of your material (like a fatty acid) while tracking how much energy it takes to make it melt. The melting point is the temperature at which a solid turns into a liquid.
So, when we’re talking about the melting point of a pure fatty acid determined by DSC, we’re really looking at the temperature at which that specific fatty acid transitions from a solid to a liquid.
Now, DSC is very precise, but even with a perfect pure fatty acid, there can be a tiny variation in its melting point, usually within about 1°C. This small range is due to factors like the purity of the sample and the conditions of the DSC experiment.
Besides the melting point, DSC also provides other valuable information about fatty acids, such as:
Heat Flow: This tells us how much energy is absorbed or released during the melting process.
Melting Onset Temperature: This is the temperature at which the melting process begins.
Knowing these additional details helps scientists better understand the properties and behavior of fatty acids, which is important for many applications, including food science, pharmaceuticals, and cosmetics.
Which fatty acid is more liquid or solid at room temperature?
The length of a fatty acid chain plays a big role in whether it’s liquid or solid. Imagine a fatty acid like a long chain. The longer the chain, the more tightly the molecules can pack together, making it harder to break them apart and turn them into a liquid.
Think about it this way: Short-chain fatty acids are like a bunch of small, wiggly worms that can’t hold onto each other very well. They’re loose and move around easily, making them more likely to be liquid at room temperature. But long-chain fatty acids are like long, strong ropes that can twist and tangle together, making them more likely to be solid.
So, the bottom line is: short-chain fatty acids are more likely to be liquid at room temperature, while long-chain fatty acids are more likely to be solid.
Let’s delve a little deeper into this. Imagine those long-chain fatty acids as a bunch of ropes that can get tangled up. The more tangled they are, the harder it is to separate them. This is similar to how the degree of saturation in a fatty acid chain affects its state at room temperature.
Saturated fatty acids are like those ropes that have no kinks or bends, making them straight and able to pack tightly together. They’re like those perfectly organized strands of yarn that are easy to weave together. This tight packing means they have stronger interactions and require more energy to break apart, making them more likely to be solid at room temperature.
Unsaturated fatty acids, on the other hand, are like those ropes with kinks or bends. These kinks are called double bonds, and they prevent the molecules from packing as tightly together. Think of them like those tangled balls of yarn that you just can’t seem to unravel. They’re less organized, making them more fluid and more likely to be liquid at room temperature.
So, to recap:
Shorter fatty acid chains are more likely to be liquid at room temperature because they can’t pack together as tightly.
Longer fatty acid chains are more likely to be solid at room temperature because they can pack together tightly.
Saturated fatty acids are more likely to be solid at room temperature because they can pack tightly together due to their straight structure.
Unsaturated fatty acids are more likely to be liquid at room temperature because their kinks and bends prevent them from packing tightly together.
Why do unsaturated fatty acids have lower melting points?
Unsaturated fatty acids have double bonds in their hydrocarbon chains. These double bonds create kinks or bends in the molecule. These kinks make it harder for the molecules to pack tightly together, which weakens the intermolecular forces between them. Think of it like trying to stack a bunch of bent straws – it’s not as easy as stacking straight straws.
Since the intermolecular forces are weaker, it takes less energy to break them apart, which means unsaturated fatty acids have lower melting points than their saturated counterparts. You’ll often find them in liquid form at room temperature.
Let’s break this down a little further.
Imagine a bunch of straight, rigid sticks. They can pack together very tightly, forming strong bonds and a solid structure. Now imagine trying to pack a bunch of those same sticks, but some of them are bent. The bent sticks make it harder to form a tight, compact structure, right? The same principle applies to fatty acids.
Saturated fatty acids are like those straight sticks. They can pack together tightly, forming a solid structure. Unsaturated fatty acids are like the bent sticks. They have kinks in their structure, making it more difficult to pack together tightly. This looser packing results in weaker intermolecular forces and a lower melting point.
Think of it like this: saturated fatty acids are like a neatly stacked pile of firewood – they’re solid and stable. Unsaturated fatty acids are like a loose pile of branches – they’re more flexible and less stable.
See more new information: bmxracingthailand.com
Which Fatty Acid Has The Lowest Melting Point?
Well, the answer is linolenic acid. It’s a polyunsaturated fatty acid, meaning it has more than one double bond in its structure. Let’s break down why that’s important.
Think of it like this: fatty acids are like long chains, and the double bonds are like kinks in the chain. The more kinks there are, the harder it is for the chains to pack tightly together.
This packing is crucial for melting point. The more tightly packed the chains are, the stronger the forces holding them together, and the higher the melting point. With linolenic acid, those double bonds make it really hard for the molecules to get close, so it melts at a very low temperature.
It’s kind of like trying to stack a bunch of twisty straws together – they just don’t fit nicely!
Understanding Melting Points
To understand why linolenic acid has the lowest melting point, we need to look at a few key factors:
Chain Length: The longer the chain, the more surface area there is for intermolecular interactions, leading to a higher melting point. Shorter chains have less surface area and therefore lower melting points.
Saturated vs. Unsaturated: Saturated fatty acids have no double bonds, meaning their chains are straight and can pack tightly together. Unsaturated fatty acids have double bonds, introducing kinks and making it harder to pack tightly. The more double bonds there are, the lower the melting point.
Cis vs. Trans: Cis fatty acids have their hydrogen atoms on the same side of the double bond, creating a sharp bend in the chain. Trans fatty acids have their hydrogen atoms on opposite sides of the double bond, creating a more linear shape. Cis fatty acids have lower melting points than trans fatty acids.
Melting Points of Other Fatty Acids
Let’s compare the melting points of some common fatty acids to see how linolenic acid stacks up:
| Fatty Acid | Melting Point (°C) | Saturation | Number of Double Bonds | Cis/Trans |
|—|—|—|—|—|
| Stearic Acid | 69.6 | Saturated | 0 | N/A |
| Palmitic Acid | 63.1 | Saturated | 0 | N/A |
| Oleic Acid | 13.4 | Monounsaturated | 1 | Cis |
| Linoleic Acid | -5 | Polyunsaturated | 2 | Cis |
| Linolenic Acid | -11 | Polyunsaturated | 3 | Cis |
As you can see, linolenic acid, with its three double bonds and cis configuration, has the lowest melting point.
The Impact of Melting Point
The melting point of fatty acids is important for many reasons:
Food Science: The melting point of fats and oils influences their texture and consistency at different temperatures. For example, fats with lower melting points are typically liquid at room temperature, making them ideal for cooking oils.
Health: Unsaturated fatty acids, particularly those with lower melting points like linolenic acid, are considered to be healthier fats. They can help lower cholesterol levels and reduce the risk of heart disease.
Industrial Applications: Fatty acids with specific melting points are used in a variety of industrial applications, such as manufacturing cosmetics, soaps, and biofuels.
FAQs
Q: Why is linolenic acid important for human health?
A: Linolenic acid is an omega-3 fatty acid, and it’s an essential fatty acid, meaning our bodies can’t make it. We need to get it from our diet. Omega-3 fatty acids play an important role in brain function, heart health, and vision.
Q: What are some good sources of linolenic acid?
A: Good sources of linolenic acid include flaxseeds, chia seeds, walnuts, and fatty fish like salmon, mackerel, and tuna.
Q: What are some other factors that can affect the melting point of fatty acids?
A: Other factors that can affect the melting point of fatty acids include:
Branching: Branched fatty acids have lower melting points than straight-chain fatty acids.
Molecular Weight: Higher molecular weight fatty acids generally have higher melting points.
Pressure: Higher pressure can increase the melting point.
Q: What is the melting point of a fatty acid used in a typical cooking oil?
A: Cooking oils are typically made from a blend of different fatty acids. The exact melting point of the oil will depend on the specific blend, but it will generally be below room temperature so that the oil is liquid at room temperature.
Q: Can I change the melting point of a fatty acid?
A: You can change the melting point of a fatty acid by modifying its structure, such as by:
Hydrogenation: Adding hydrogen to double bonds can saturate the fatty acid, raising its melting point.
Interesterification: Rearranging the fatty acids on a glycerol molecule can change the overall melting point of the fat.
Q: Why does knowing the melting point of a fatty acid matter?
A: The melting point is a key property that influences the behavior and applications of fatty acids. It’s important for understanding how fats and oils behave in food, in our bodies, and in industrial processes.
So there you have it! The next time you’re thinking about fats and oils, remember that linolenic acid is the champion of low melting points.
10.15: Lipids—Part 2 – Chemistry LibreTexts
Melting Points of Saturated vs. Unsaturated Fatty Acids. Note that as a group, the unsaturated fatty acids have lower melting points than the saturated fatty acids. The Chemistry LibreTexts
Solved Which of the following fatty acids has the lowest – Chegg
Science. Chemistry questions and answers. Which of the following fatty acids has the lowest melting point? Linoleic acid Palmitoleic acid Arachidonic acid Oleic acid. Your Chegg
2.32 Fatty Acids | Nutrition – Lumen Learning
As a result, the melting point is much lower for cis fatty acids compared to trans and saturated fatty acids. To illustrate this difference, the figure below shows the difference in the melting points of saturated, trans-, Lumen Learning
2.7: Fatty Acids – Biology LibreTexts
Mixtures with higher percentages of saturated fatty acids have a higher melting point and if they are solid at room temperature, they are referred to as fats. Triacylglycerol mixtures remaining liquid at room Biology LibreTexts
SOLVED: Arrange the fatty acids from highest melting point to
Arrange the fatty acids from highest melting point to lowest melting point. Highest melting point. CH3(CH2)COOH. CH3(CH2)2COOH. Numerade
A Comprehensive Evaluation of the Melting Points of
The melting point of a pure fatty acid or ester as determined by DSC can vary up to approximately 1 °C. Other thermal data, including heat flow and melting onset temperatures are briefly AOCS Publications
Lipids and Membrane Structure and Transport Flashcards | Quizlet
Free fatty acids are transported in blood complexed to albumin. Stearic acid is 18:0 and has a higher melting point than palmitic acid which is 16:0. Concept: the melting point Quizlet
The lowest melting point of an essential fatty acid is that of – Toppr
The lowest melting point of an essential fatty acid is that of arachidonic acid, which is of -49°C. The melting points of linoleic acid and linolenic acid are -5°C and -11°C. So, the Toppr
SOLVED: Which fatty acid would you expect to have the lowest
Fatty acids with shorter carbon chains and fewer double bonds tend to have lower melting points. Option A has a carbon chain length of 8 and one double bond, while option B has Numerade
Melting Points Of Fatty Acids
Rank The Following Fatty Acids From Highest Melting Point To Lowest Melting Point
Why Stearic Acid Has More Melting Point Than Oleic Acid?
Melting Point Of Saturated\U0026 Unsaturated Fatty Acid
Saturated Vs Unsaturated Fatty Acids – Chemical Structure Explained
The Lowest Melting Point Of An Essential Fatty Acid Is That Of
Rank These Fatty Acids In Order Of Their Melting…
Rank These Fatty Acids In Order Of Their Melting…
Chapter 15 Exercise 4 Explaining The Different Melting Points Of Different Fatty Acids
Does 100% Pure Water Have A Taste? Drinking Type Ii Deionized Water Experiment
Link to this article: which fatty acid has the lowest melting point.
See more articles in the same category here: bmxracingthailand.com/what
