What is the principle of cold vapour technique?
Here’s how it works:
1. Sample Preparation: The sample containing mercury is treated with a reducing agent, such as stannous chloride (SnCl2), to convert any mercury ions (Hg2+) to elemental mercury (Hg0).
2. Aeration: The solution is then aerated, which means bubbling air through it. This process drives the elemental mercury into the gas phase.
3. Vapor Transport: The mercury vapor is transported through a tube into a quartz cell positioned in the light path of an atomic absorption spectrophotometer.
4. Atomic Absorption: The spectrophotometer emits a beam of ultraviolet light with a specific wavelength that is absorbed by mercury atoms in the vapor. The amount of light absorbed is directly proportional to the concentration of mercury in the sample.
Why Cold Vapor?
The cold vapor technique offers several advantages over other methods for mercury analysis.
High Sensitivity: The technique is incredibly sensitive, allowing for the detection of very low levels of mercury. This is crucial for environmental monitoring, where even small amounts of mercury can pose significant health risks.
Simplicity: The procedure is relatively straightforward and can be easily automated, making it a practical choice for routine analysis.
Versatility: The cold vapor technique can be applied to analyze mercury in various matrices, including water, soil, biological samples, and air.
The cold vapor technique is a powerful tool for accurately and efficiently measuring mercury in a wide range of samples. Its high sensitivity, simplicity, and versatility make it a valuable technique in environmental monitoring, food safety, and industrial applications.
What is the principle and instrumentation of atomic absorption spectroscopy?
Imagine an atom like a tiny, unique fingerprint. Each element has its own distinct set of wavelengths that it can absorb. When you shine a beam of light containing those specific wavelengths through a sample containing the element, the atoms will absorb some of the light. This absorption is directly proportional to the concentration of the element in the sample.
Let’s break it down further:
Atoms absorb light: When a specific wavelength of light is shone on an atom, the atom can absorb the energy from that light.
Unique wavelengths: Every element has its own unique set of wavelengths that it absorbs. This is like a unique fingerprint for each element.
Concentration and Absorption: The amount of light absorbed by the sample is directly proportional to the concentration of the element in the sample.
This is the heart of atomic absorption spectroscopy. Think of it like shining a flashlight through a dense fog. The more fog there is (the higher the concentration of fog particles), the less light will shine through. In AAS, the more of a specific element is present in the sample, the less light will pass through, allowing us to determine its concentration.
What is the atomic absorption of mercury?
Mercury absorbs 254-nm light in direct proportion to its concentration in a sample. This phenomenon is the basis of a powerful analytical technique called cold vapor atomic absorption spectrometry.
To understand this, imagine shining a beam of 254-nm light through a sample containing mercury. As the light passes through the sample, mercury atoms absorb some of it. The amount of light absorbed is directly proportional to the number of mercury atoms present.
Here’s a simplified breakdown:
More mercury in the sample means more light gets absorbed.
Less mercury in the sample means less light gets absorbed.
This relationship forms the core of cold vapor atomic absorption spectrometry, allowing us to precisely measure the amount of mercury in various samples.
Cold vapor atomic absorption spectrometry is a sensitive and specific technique used in many applications, including:
Environmental monitoring to assess mercury levels in water, air, and soil.
Food safety to determine mercury levels in fish and other seafood.
Clinical chemistry to measure mercury levels in blood and urine.
Cold vapor atomic absorption spectrometry is a crucial tool for understanding and managing mercury in our environment and ensuring our health and safety.
What is cold vapour atomic absorption spectroscopy for HG?
Let’s break down how this method works:
1. Sample Preparation: The first step involves preparing the sample by adding a reducing agent like stannous chloride (SnCl2) to the water sample. This converts the mercury ions in the water to elemental mercury vapor.
2. Vapor Generation: The elemental mercury vapor is then generated in a closed system. This system typically involves bubbling an inert gas, like nitrogen, through the sample solution. The mercury vapor is carried by the gas stream into an absorption cell.
3. Absorption Cell: The absorption cell contains a beam of ultraviolet (UV) light. The mercury vapor absorbs the UV light at a specific wavelength, which is characteristic of mercury.
4. Detection: A detector measures the amount of UV light absorbed by the mercury vapor. The amount of absorption is directly proportional to the concentration of mercury in the sample.
The CVAA technique is highly sensitive, making it ideal for detecting even trace amounts of mercury in water samples. It’s also a relatively straightforward method, requiring minimal sample preparation.
What is cold vapor analysis method?
CVAAS works by converting mercury in the sample to atomic mercury vapor, which is then measured by atomic absorption spectroscopy. The process begins by reducing the mercury in the sample to elemental mercury using a reducing agent. This elemental mercury then gets converted to atomic mercury vapor by bubbling an inert gas, such as argon, through the sample. The atomic mercury vapor then passes through a beam of ultraviolet light, and the amount of light absorbed is directly proportional to the concentration of mercury in the sample. This method is incredibly sensitive and can detect mercury at concentrations as low as parts per billion.
CVAAS is widely used in a variety of applications, including:
Monitoring mercury levels in drinking water
Analyzing mercury in environmental samples
Measuring mercury in biological samples
Assessing mercury contamination in food
Because of its accuracy, sensitivity, and ease of use, CVAAS remains the most reliable method for measuring mercury levels in various settings.
What are the instruments used in AAS?
Atomic Absorption Spectrometry (AAS) is a powerful analytical technique used to determine the concentration of specific elements in a sample. It’s widely used in various fields like environmental monitoring, food safety, and clinical chemistry.
The heart of any AAS system lies in its four key components:
The sample introduction area: This is where the sample is introduced into the instrument. The sample can be in liquid, solid, or gaseous form, and it’s crucial to introduce it accurately and efficiently.
The light (radiation) source: This component emits a specific wavelength of light that corresponds to the element being analyzed.
The monochromator or polychromator: This acts as a filter, allowing only the desired wavelength of light to pass through.
The detector: This component measures the amount of light that passes through the sample.
These components work together to measure the absorbance of light by the atoms of the analyte in the sample. This absorbance is directly proportional to the concentration of the analyte, making AAS a reliable method for quantitative analysis.
Delving Deeper into the Components
Now let’s break down each component to understand their roles better:
1. Sample Introduction Area
The goal: To introduce the sample into the AAS system in a controlled and reproducible manner.
The players: Common techniques include flame atomization, electrothermal atomization, and hydride generation.
Flame atomization: The sample is aspirated into a flame, where it is atomized (converted into free atoms).
Electrothermal atomization: The sample is placed in a graphite furnace and heated to a high temperature, causing atomization.
Hydride generation: This technique is used for volatile elements like arsenic and selenium. The sample is reacted with a reducing agent to form a volatile hydride, which is then transported to the atomizer.
2. Light (Radiation) Source
The goal: To generate light at the specific wavelength that is absorbed by the analyte.
The players: Hollow cathode lamps (HCLs) are the most common light source in AAS. These lamps contain a cathode made of the element being analyzed. When an electric current is passed through the lamp, the element in the cathode is excited and emits light at its characteristic wavelengths.
3. Monochromator or Polychromator
The goal: To isolate the desired wavelength of light emitted by the hollow cathode lamp and pass it through the sample.
The players:
Monochromators: These use a diffraction grating to separate different wavelengths of light.
Polychromators: These use multiple detectors to simultaneously measure the absorbance at several wavelengths.
4. Detector
The goal: To measure the intensity of the light beam after it passes through the sample.
The players: Photomultiplier tubes are the most common detectors used in AAS. These devices convert photons of light into electrical signals that are proportional to the intensity of the light beam.
Together, these components provide a robust and efficient system for quantitative analysis of elements in a variety of sample types.
What is the principle and instrumentation of atomic emission spectroscopy?
How it Works:
Think of atoms like tiny light bulbs that can be turned on and off. In AES, we excite the atoms in a sample by providing them with energy. This energy causes the atoms to jump to a higher energy level, becoming “excited.” Like a light bulb, these excited atoms don’t stay in this energized state for long. They quickly return to their ground state, releasing the excess energy as light.
The wavelength of the emitted light is specific to the element, kind of like a fingerprint that identifies the atom. We use a spectrometer to measure these wavelengths, and by analyzing the intensity of the emitted light, we can determine the concentration of the element in the sample.
The Setup:
To get this process going, we need a few key components:
1. Sample Introduction: First, we introduce the sample into the instrument. The sample could be a solid, liquid, or gas.
2. Excitation Source: The heart of the AES system is the excitation source. This source provides the energy needed to excite the atoms. We use a plasma or a flame for this purpose.
– Plasma: A plasma is a super-heated, ionized gas. It’s like a really hot soup of charged particles that can effectively energize atoms.
– Flame: A flame provides a less intense but still effective way to excite atoms. The heat of the flame provides the energy for atomic excitation.
3. Spectrometer: The spectrometer is like a prism that separates the emitted light based on its wavelength. It allows us to measure the intensity of the light at specific wavelengths, which is directly related to the concentration of the element in the sample.
4. Detector: Finally, a detector converts the light signal into an electrical signal that can be processed and displayed as a spectrum. The intensity of each spectral line is proportional to the concentration of the corresponding element in the sample.
In summary, AES is a method that excites atoms to emit light at specific wavelengths, and the intensity of this emitted light is proportional to the concentration of the element in the sample. This process involves vaporizing the sample, exciting the atoms with a plasma or flame, and then analyzing the emitted light using a spectrometer. The resulting spectrum provides information about the elemental composition of the sample.
What is spectroscopy instrumentation techniques?
Several common spectroscopy techniques are widely used in various scientific fields, including:
Infrared (IR) Spectroscopy: This technique is used to identify functional groups within a molecule by analyzing the absorption of infrared radiation.
Ultraviolet-Visible (UV-Vis) Spectroscopy: This method measures the absorption and transmission of ultraviolet and visible light by a sample. It helps determine the concentration of a substance or analyze its electronic structure.
Atomic Absorption Spectroscopy (AAS): AAS is a sensitive technique used to determine the elemental composition of a sample. It measures the absorption of light by atoms in the sample.
Raman Spectroscopy: This technique analyzes the scattering of light by molecules. Raman spectroscopy provides information about the vibrational modes of molecules, allowing for the identification of specific molecules or functional groups.
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy is a powerful technique for determining the structure and dynamics of molecules. It exploits the magnetic properties of atomic nuclei to obtain information about the arrangement of atoms in a molecule.
Electron Spectroscopy: Electron spectroscopy involves analyzing the energy of electrons emitted or absorbed by a sample. Techniques like X-ray photoelectron spectroscopy (XPS) can provide information about the elemental composition, chemical states, and electronic structure of materials.
Mass Spectrometry (MS): MS is a technique that measures the mass-to-charge ratio of ions. It can identify and quantify different molecules in a sample, providing information about their molecular weight, structure, and abundance.
Each of these spectroscopic techniques plays a vital role in different fields. For example, IR spectroscopy is commonly used in the pharmaceutical industry to identify and characterize drugs, while UV-Vis spectroscopy is widely applied in the food industry for quality control and analysis. NMR spectroscopy is crucial in drug discovery and development, while mass spectrometry is used in environmental monitoring and forensic science.
See more here: What Is The Principle And Instrumentation Of Atomic Absorption Spectroscopy? | Principle And Instrumentation Of Cold Vapour Aas
What is cold vapor atomic absorption spectroscopy (CVAA)?
CVAA is a highly sensitive method for measuring mercury in various samples, including environmental samples, biological samples, and industrial materials. The technique relies on the principle of atomic absorption spectroscopy, where a beam of light is passed through a sample vaporized in a specific environment. The atoms of the element being measured, in this case, mercury, absorb the light at a specific wavelength. The amount of light absorbed is directly proportional to the concentration of the mercury atoms in the vapor.
Here’s how CVAA works:
1. Sample Preparation: The sample containing mercury is first treated to convert mercury into its elemental form (Hg). This is often done by adding a reducing agent like stannous chloride (SnCl2) to the sample.
2. Generation of Cold Vapor: The prepared sample is then introduced into a reaction vessel where it is reacted with a reducing agent to produce mercury vapor. This vapor is then passed through a flow cell.
3. Atomic Absorption Measurement: A beam of light from a mercury lamp is passed through the flow cell containing the mercury vapor. The mercury atoms in the vapor absorb specific wavelengths of light, causing a decrease in the intensity of the light beam.
4. Signal Detection: The decrease in light intensity is measured by a detector, and the signal is directly proportional to the concentration of mercury in the sample.
CVAA is highly sensitive, capable of detecting mercury concentrations as low as a few nanograms per liter. This makes it a valuable tool for monitoring mercury levels in various applications, such as environmental monitoring, food safety, and industrial hygiene. The technique offers advantages over other methods for mercury analysis, including its high sensitivity, relative simplicity, and cost-effectiveness.
What is a cold vapor atomic absorption system?
A CVAAS uses a clever technique to measure mercury levels. It starts with a peristaltic pump that delivers your sample and a solution called stannous chloride into a special device called a gas-liquid separator. This separator is where the magic happens! A stream of pure, dry gas, like argon or nitrogen, is bubbled through the mixture. This bubbling action causes the mercury in your sample to transform into mercury vapor, which is then measured by the system.
Here’s a breakdown of how it works:
Sample Introduction: The sample, which may contain mercury, is introduced into the system.
Chemical Reduction: The stannous chloride acts as a reducing agent, converting any mercury ions present in the sample into elemental mercury.
Vapor Generation: The bubbling gas carries the mercury vapor from the liquid phase to the gas phase.
Atomic Absorption: The mercury vapor then passes through a beam of light from a mercury lamp. Atoms of mercury in the vapor absorb some of the light, which is then measured by a detector.
Mercury Concentration: The amount of light absorbed is directly proportional to the concentration of mercury in the original sample.
This process is highly sensitive, allowing CVAAS to detect even trace amounts of mercury in various samples, including environmental samples like water, soil, and air, as well as biological samples like blood and urine.
Think of it this way: CVAAS is like a super-powered magnifying glass for mercury. It allows us to see even the tiniest amounts of this heavy metal, ensuring our safety and environmental protection.
What is the cold vapor method?
In this method, a sample containing mercury is reacted with stannous chloride in a vessel outside of the Atomic Absorption (AA) instrument. This reaction creates atomic mercury vapor. The vapor is then carried by a stream of inert gas, like argon, to the AA instrument. Here, the mercury atoms absorb ultraviolet light at a specific wavelength. This absorption is measured, and the signal is proportional to the amount of mercury in the sample.
The cold vapor method has several advantages over other methods for measuring mercury. One advantage is that it is very sensitive and can detect even low levels of mercury. Another advantage is that it is relatively simple and can be carried out in a standard laboratory. However, it’s important to be aware that some aspects of this method require specialized equipment and technical expertise.
The method involves a chemical reaction that reduces mercury in the sample to atomic mercury. This reaction is typically performed in a vessel called a reaction vessel, which is connected to the AA instrument. The reaction is carried out by adding a solution of stannous chloride to the sample. The stannous chloride reduces the mercury in the sample to atomic mercury.
The atomic mercury vapor is then carried to the AA instrument by a stream of inert gas. This gas stream typically flows through a gas-liquid separator, which removes any liquid droplets from the gas stream. The gas-liquid separator is essential for ensuring that only the atomic mercury vapor reaches the AA instrument.
The AA instrument uses a beam of ultraviolet light to excite the mercury atoms in the vapor. When the mercury atoms are excited, they absorb light at a specific wavelength. The amount of light absorbed is proportional to the concentration of mercury in the vapor.
The cold vapor method is a powerful tool for measuring mercury in a wide variety of samples. It is used in many applications, such as environmental monitoring, food safety, and industrial hygiene.
What is the difference between Flame AA and cold vapor technique?
Cold vapor atomic absorption spectrometry (CVAAS) is much more sensitive than FAAS for mercury analysis. This means that CVAAS can detect much lower concentrations of mercury than FAAS. In fact, CVAAS is about four orders of magnitude more sensitive. This is a huge difference!
To put this into perspective, the U.S. Environmental Protection Agency (EPA) has set a limit for mercury in drinking water of 2 µg/L, or 2 parts per billion (ppb). CVAAS is the only EPA-approved method for measuring mercury at these low levels.
Why is CVAAS so much more sensitive? Here’s the deal:
FAAS works by atomizing a sample in a flame. The flame excites the mercury atoms, causing them to absorb light at a specific wavelength. The amount of light absorbed is proportional to the concentration of mercury in the sample.
CVAAS is different because it uses a chemical reaction to convert mercury ions into elemental mercury vapor. This vapor is then passed through a cell where it absorbs light at a specific wavelength. This process is much more efficient at converting mercury into a detectable form, which makes CVAAS much more sensitive.
CVAAS is the gold standard for measuring low levels of mercury in a variety of samples, including water, soil, and biological samples. It is an incredibly powerful tool for environmental monitoring, public health, and safety.
See more new information: bmxracingthailand.com
Principle And Instrumentation Of Cold Vapour Aas | What Is The Principle Of Cold Vapour Technique?
Let’s delve into the world of cold vapor atomic absorption spectrometry (CV-AAS), a powerful analytical technique used to determine the concentration of certain elements, primarily mercury (Hg), in various samples.
The Principle Behind CV-AAS
CV-AAS leverages the principle of atomic absorption spectrometry (AAS), a technique where a beam of light from a hollow cathode lamp (HCL) passes through a sample containing the analyte of interest. If the sample contains the analyte, atoms in the ground state will absorb the light at specific wavelengths, causing a reduction in the light intensity reaching the detector.
But here’s the catch – not all elements are easily analyzed using traditional AAS methods. Mercury, for example, is a volatile element and can easily be lost during the atomization process. This is where CV-AAS comes into play.
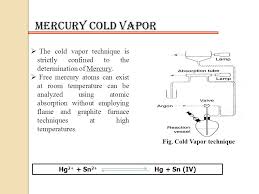
In CV-AAS, the sample is first treated with a reducing agent, typically tin(II) chloride (SnCl2), in an acidic medium. This reaction converts the mercury ions (Hg2+) in the sample to elemental mercury (Hg). The generated elemental mercury is then purged from the solution using an inert gas like nitrogen or argon and transported to a quartz cell placed in the path of the light beam.
The heart of this technique lies in this gas-phase atomization process. Here, mercury atoms absorb the light from the mercury HCL, leading to a decrease in light intensity detected. This decrease in intensity is proportional to the concentration of mercury in the original sample.
Instrumentation of CV-AAS
The instrumentation of a typical CV-AAS system involves several key components:
1. Sample Introduction System: This system handles the introduction of the sample into the CV-AAS apparatus. It includes a sample vial, a peristaltic pump for delivering the sample to a reaction vessel, and a reagent delivery system for adding reducing agents and acids.
2. Reaction Vessel: This is where the chemical reaction takes place, converting mercury ions to elemental mercury. This reaction is typically carried out in a closed vessel to ensure efficient trapping and transfer of mercury vapor.
3. Gas Flow System: The gas flow system provides a controlled flow of inert gas, typically nitrogen or argon, for purging and transporting the generated mercury vapor.
4. Quartz Cell: The quartz cell is placed in the path of the light beam from the mercury HCL. This cell acts as an absorption cell, where the mercury atoms absorb the light at specific wavelengths.
5. Hollow Cathode Lamp (HCL): The HCL serves as the light source for the technique. It emits light at specific wavelengths corresponding to the mercury atoms.
6. Detector: A photomultiplier tube (PMT) or a similar type of detector is used to measure the intensity of the light that passes through the quartz cell.
7. Electronics and Data Processing System: This system controls the instrument’s operation, collects the signal from the detector, and processes the data to determine the concentration of mercury in the sample.
Advantages of CV-AAS
CV-AAS offers several advantages over conventional AAS techniques for mercury analysis:
* High Sensitivity: CV-AAS can achieve very low detection limits for mercury, making it ideal for analyzing trace amounts of mercury in various samples.
* Simplicity: The technique is relatively simple and straightforward to perform, requiring minimal sample preparation.
* Specificity: The use of a specific mercury HCL ensures high selectivity, minimizing interference from other elements present in the sample.
* Cost-Effectiveness: CV-AAS systems are generally less expensive than other analytical techniques for mercury determination.
Applications of CV-AAS
CV-AAS finds broad applications in various fields due to its high sensitivity and specificity for mercury analysis. Some prominent applications include:
* Environmental Monitoring: CV-AAS is used to monitor mercury levels in air, water, soil, and biological samples to assess environmental contamination and potential risks to human health.
* Food Safety: CV-AAS is employed to measure mercury levels in food products, ensuring food safety and preventing mercury accumulation in the food chain.
* Industrial Hygiene: CV-AAS is used to monitor mercury exposure in workplaces, protecting workers from potential health hazards.
* Clinical Chemistry: CV-AAS is used to measure mercury levels in blood and urine samples to assess potential mercury poisoning and evaluate treatment efficacy.
* Geological Analysis: CV-AAS helps determine mercury concentrations in geological samples, providing insights into geological processes and mineral deposits.
FAQs
1. Why is CV-AAS preferred for mercury analysis compared to other AAS techniques?
CV-AAS offers higher sensitivity, enabling the detection of very low mercury concentrations, crucial for environmental and biological applications. The technique avoids the need for high temperatures, reducing the risk of mercury loss during the atomization process.
2. What are the potential interferences in CV-AAS?
Interferences in CV-AAS can arise from various sources, including:
* Chemical Interferences: Some metals, such as gold and silver, can interfere with the reduction of mercury ions to elemental mercury.
* Spectral Interferences: Other elements emitting light at similar wavelengths to mercury can interfere with the measurement.
3. How can interferences be minimized in CV-AAS?
Interferences can be minimized by using appropriate sample preparation methods, including pre-concentration techniques and chemical separation methods, and by employing specialized analytical procedures, such as standard addition methods.
4. What are the limitations of CV-AAS?
CV-AAS is primarily limited to the analysis of mercury due to the technique’s reliance on a specific mercury HCL. It also requires careful handling of mercury samples due to the element’s toxicity.
5. What are the future trends in CV-AAS?
Future developments in CV-AAS focus on improving sensitivity and automation, developing portable and miniaturized systems, and exploring applications for analyzing other volatile elements beyond mercury.
6. What are the other methods for determining mercury?
Other methods for determining mercury include:
* Atomic Fluorescence Spectrometry (AFS): AFS offers high sensitivity for mercury analysis and is often used in conjunction with CV-AAS.
* Inductively Coupled Plasma Mass Spectrometry (ICP-MS): ICP-MS provides a multi-element analytical capability and can determine mercury concentrations in various matrices.
* Cold Vapor Atomic Fluorescence Spectrometry (CVAFS): This technique combines the advantages of CV-AAS and AFS, achieving very low detection limits for mercury.
CV-AAS has become an indispensable tool for various fields, offering a reliable, sensitive, and specific method for mercury analysis. Its applications continue to expand, driven by the growing need to monitor mercury levels in our environment, food, and workplaces. As we continue to explore its potential, CV-AAS will remain a valuable analytical technique in the years to come.
What is Cold Vapor Atomic Absorption (CVAA)
Cold Vapor Atomic Absorption Spectroscopy or CVAAS is one of the primary techniques for mercury analysis. Introduced in 1968 by Hatch and Ott, CVAAS is now the reference method for drinking water monitoring teledyneleemanlabs.com
The Determination of Mercury by Cold Vapor Atomic Absorption
The cold vapor atomic absorption technique for mercury has received the greatest attention. The cold vapor principle was first proposed by Poluektov and co Agilent
Using cold vapor generation atomic absorption to determine
Instrument and software The Thermo Scientific™ iCE™ 3300 Atomic Absorption Spectrometer (AAS) was used during this analysis. The iCE 3300 AAS combines high thermofisher.com
Hydride / Cold Vapor – Chemistry LibreTexts
Cold vapor generation is the most common method for measuring trace levels of mercury. The other elements do not have significant vapor pressure at room Chemistry LibreTexts
Mercury and mercury compounds – Wiley Online Library
Mercury is determined by flow injection cold vapour atomic absorption spectrometry (CV-AAS). The digested blood or urine samples are stabilised with potassium permanganate, Wiley Online Library
Automated Cold Vapor Determination of Mercury: EPA Stannous
Introduction . The determination of Hg by cold-vapor atomic absorption was first proposed by Poluektov et. al. [1] in 1963. In this method mercuric ions in an acidic Agilent
Atomic Absorption Spectroscopy | SpringerLink
This is the basic principle of atomic absorption spectroscopy (AAS) whereas atomic fluorescence spectroscopy (AFS) is based on the re-emission of absorbed Springer
Atomic absorption spectrometry – Wiley Online Library
The chapter first describes the basis of atomic absorption spectrometry (AAS) and then discusses the main characteristics of flame atomization (FAAS), Wiley Online Library
Continuous flow cold vapor atomic absorption determination of
A continuous flow cold vapour procedure for mercury determination by atomic emission using the reverse flow injection approach. Spectrochimica Acta Part B: Atomic ACS Publications
Explain The Principle Of Atomic Absorption Spectrometer (Aas)
Cold Vapour Atomic Absorption Technique
Sampling With A Flame Aas
Quickly Understand Atomic Absorption Spectroscopy (Aas)
Graphite Furnace Atomic Absorption Spectroscopy | Principle | Instrumentation | Applictions
Aas Animation
Cold Vapour Aas-Very Easy Way.. Important Of M.Sc.Notes
Atomic Absorption Spectroscopy | Introduction \U0026 Instrumentation
Cài Đặt Phép Đo Máy Aas Thermo 3300 (Ice 3000 Series Atomic Absorption Spectrometers) – Poshaco Hy
Hydride Generation Aas And Cold Vapour Hg Aas
Link to this article: principle and instrumentation of cold vapour aas.
See more articles in the same category here: bmxracingthailand.com/what
