Is OCH3 activating?
You’re right to be curious about this. OCH3 is a common substituent in organic chemistry, and understanding its effect on aromatic rings is crucial. We know that ortho-para directors are groups that influence where an electrophile will attack on a benzene ring.
OCH3, along with CH3, OH, and NH2, is indeed an activating group. These groups are all electron-donating, meaning they increase the electron density of the benzene ring. This makes the ring more reactive towards electrophiles, hence the term “activating.”
The reason OCH3 is activating is because of the oxygen atom. Oxygen is more electronegative than carbon, so it pulls electron density away from the carbon atom it’s attached to. This electron density then gets pushed towards the benzene ring, making the ring more electron-rich.
Now, let’s dig a bit deeper into how this works.
OCH3 can donate electrons to the benzene ring in two ways:
1. Inductive effect: The oxygen atom is more electronegative than the carbon atom, so it pulls electron density away from the carbon. This effect is called the inductive effect. The carbon, now slightly positive, pulls electron density from the adjacent carbon in the benzene ring, and this effect propagates throughout the ring.
2. Resonance effect: Oxygen has lone pairs of electrons, and it can donate these electrons to the benzene ring through resonance. This creates resonance structures where the positive charge is delocalized over the ring, further increasing the electron density of the ring.
The combined effect of the inductive and resonance effects makes OCH3 a strong activating group. This means that when OCH3 is attached to a benzene ring, it makes the ring more reactive towards electrophilic substitution reactions.
For example, if you were to react a benzene ring with OCH3 attached to it with nitric acid (HNO3), the OCH3 group would direct the electrophile (NO2+) to the ortho and para positions on the ring, and the reaction would occur more readily than with an unsubstituted benzene ring.
So, to summarize, OCH3 is an activating group because it donates electrons to the benzene ring through inductive and resonance effects. This makes the ring more electron-rich and more reactive towards electrophilic substitution reactions.
Is OCH3 electron donating or withdrawing?
Let’s clear up the misconception: The methoxy group is actually electron donating, not electron withdrawing.
Here’s why:
While oxygen is indeed more electronegative than carbon, the inductive effect isn’t the whole story. The resonance effect plays a much more significant role in determining the overall electron-donating or -withdrawing nature of the methoxy group.
The lone pairs of electrons on the oxygen atom in the methoxy group can participate in resonance with the pi system of the aromatic ring. This delocalization of electrons creates a partial negative charge on the ring and a partial positive charge on the oxygen atom. This effectively pushes electron density into the aromatic ring, making the methoxy group electron donating.
Think of it this way: The oxygen atom, with its lone pairs, is like a “pushing” force, donating electrons to the ring. This “push” is more powerful than the “pull” of the oxygen atom’s electronegativity via the inductive effect.
To further solidify this concept, consider the Hammett substituent constant (σm), which is a quantitative measure of the electron-donating or -withdrawing ability of a substituent. For the methoxy group, σm is negative (-0.27), indicating its electron-donating nature.
In summary, the methoxy group (-OCH3) is an electron-donating group due to its resonance effect, which outweighs the inductive effect. This electron-donating nature plays a significant role in influencing the reactivity of molecules containing the methoxy group.
Is methoxy activating or deactivating?
Let’s look at anisole (methoxybenzene) as an example. When the lone pair of electrons on the oxygen atom in the methoxy group participates in resonance with the benzene ring, it creates a negative charge at the *ortho* and *para* positions of the ring. This negative charge makes these positions more susceptible to attack by electrophiles.
Why is the methoxy group an activating group?
The methoxy group is considered an activating group because it increases the electron density of the benzene ring, making it more reactive towards electrophilic attack. Here’s a more detailed explanation:
1. Electron-donating nature: The oxygen atom in the methoxy group has two lone pairs of electrons. These lone pairs can participate in resonance with the benzene ring. This delocalization of electrons makes the ring more electron-rich.
2. Resonance stabilization: When the methoxy group participates in resonance, it forms a resonance structure where a negative charge is located at the *ortho* and *para* positions of the benzene ring. This negative charge makes the ring more susceptible to attack by electrophiles.
3. Stabilization of the carbocation intermediate: During electrophilic aromatic substitution, a carbocation intermediate is formed. The electron-donating nature of the methoxy group helps stabilize this carbocation intermediate by delocalizing the positive charge. This stabilization lowers the activation energy of the reaction and increases the rate of reaction.
Therefore, the methoxy group is an activating group because it increases the electron density of the benzene ring, making it more reactive towards electrophilic attack. This is a key factor in understanding the reactivity of aromatic compounds in organic chemistry.
Is a ketone activating or deactivating?
Why do ketones deactivate aromatic rings? It’s all about electron density. Ketones have a double bond between a carbon and an oxygen atom. Oxygen is more electronegative than carbon, meaning it attracts electrons more strongly. This pulls electron density away from the aromatic ring, making it less likely to react with electrophiles.
Let’s look at resonance to understand this better. Resonance is a way to represent the delocalization of electrons in a molecule. When a ketone is attached to an aromatic ring, we can draw resonance structures where the electrons from the ring are delocalized onto the oxygen atom of the ketone. These resonance structures show that the electron density is being pulled away from the aromatic ring, towards the ketone.
This decrease in electron density makes the aromatic ring less reactive towards electrophilic attack. Think of it like this: the ring is less likely to share its electrons with an electrophile because they’re already being pulled away by the ketone. This makes the ring less likely to undergo reactions like nitration, halogenation, and Friedel-Crafts alkylation.
Here’s a summary of how ketones deactivate aromatic rings:
Electron withdrawing effect: The oxygen atom in the ketone pulls electron density away from the ring.
Resonance: Resonance structures show how the electron density is delocalized onto the oxygen atom, further reducing the electron density in the ring.
Less reactive: The decreased electron density makes the ring less reactive towards electrophilic aromatic substitution.
Now, you might be wondering if there are any groups that activate aromatic rings. Absolutely! There are groups like alkyl groups, alkoxy groups, and amines that donate electron density to the ring, making it more reactive towards electrophiles.
Is amide activating or deactivating?
Here’s a visual breakdown:
Amide Group
* The nitrogen atom has a lone pair of electrons.
* These electrons can be delocalized (spread out) by resonance.
* This resonance can happen in two directions:
Towards the ring: This is what makes the amide activating.
Towards the carbonyl group: This reduces the electron density available for activation.
Amino Group
* The nitrogen atom has a lone pair of electrons.
* These electrons can be delocalized (spread out) by resonance.
* There’s only one direction for this resonance:
Towards the ring: This is what makes the amino group strongly activating.
The amide group is less activating than the amino group because the competition for the nitrogen’s lone pair electrons weakens its ability to donate electrons to the ring.
Think of it like this: Imagine you have a friend who’s trying to share their toys with two different kids. They’re less likely to share as much with each kid because they’re trying to split their time and attention between them. The nitrogen lone pair in the amide group is similar – it’s trying to share its electrons with both the ring and the carbonyl group, making it less effective at activating the ring.
Is CH3 activating or deactivating?
Let’s delve deeper into why CH3 is an activating group. It all boils down to the electron-donating nature of the methyl group. The carbon atom in CH3 is sp3 hybridized, making it slightly more electron-rich than the sp2 hybridized carbon atoms in the benzene ring. This electron density from the methyl group can be delocalized into the benzene ring through resonance. The resonance structures contribute to a higher electron density in the ring, making it more susceptible to attack by electrophiles. Think of it like this: a higher electron density in the ring makes it more attractive to positively charged electrophiles, leading to a faster reaction.
Here’s a simple analogy: Imagine the benzene ring as a sponge that can absorb water. The CH3 group acts like a water source, making the sponge more absorbent. The electrophile is like a thirsty person looking for water. A more absorbent sponge will attract the thirsty person faster, just like a more electron-rich benzene ring will react faster with an electrophile.
See more here: Is Och3 Electron Donating Or Withdrawing? | Is Och3 Activating Or Deactivating
What is the difference between -OCH3 and -CH3?
Both groups influence the electron density of the aromatic ring, but in different ways. -CH3 is an electron-donating group due to its +I effect, meaning it pushes electron density towards the ring. This makes the ring more reactive towards electrophilic aromatic substitution reactions. On the other hand, -OCH3 is an electron-withdrawing group due to its -I effect. This means it pulls electron density away from the ring, making it less reactive towards electrophilic substitution.
However, the story doesn’t end there. -OCH3 also exhibits a significant +R effect (resonance effect) due to the lone pairs of electrons on the oxygen atom. This effect dominates the -I effect and makes -OCH3 an overall electron-donating group. This is why both -OCH3 and -CH3 are considered activating groups for the benzene ring, meaning they make the ring more reactive towards electrophilic substitution reactions.
Now, you might wonder why both -OCH3 and -CH3 make the ortho and para positions on the benzene ring equally electron-rich. This is because of the resonance effect. The lone pairs of electrons on the oxygen atom in -OCH3 can delocalize into the benzene ring, creating resonance structures where the positive charge is distributed on the ortho and para positions. This makes these positions more electron-rich and susceptible to electrophilic attack. Similarly, the -CH3 group can also participate in hyperconjugation, where the C-H bonds in the methyl group can donate electron density to the benzene ring, again favoring the ortho and para positions.
Here’s a summary:
-CH3
+I effect: Donates electrons, making the ring more reactive.
Hyperconjugation: Donates electron density to the ring, favoring ortho and para positions.
-OCH3
-I effect: Withdraws electrons, making the ring less reactive.
+R effect: Dominates the -I effect, making it an overall electron-donating group.
Resonance: Delocalizes electrons, making the ortho and para positions more electron-rich.
So, both -CH3 and -OCH3 are considered activating groups because they increase the electron density in the benzene ring, making it more reactive towards electrophilic substitution reactions. While both groups activate the ring, -OCH3 is considered a stronger activator due to the combined effects of its +R effect and -I effect. This means that a benzene ring with an -OCH3 group will be more reactive towards electrophilic substitution reactions than a benzene ring with a -CH3 group.
Is Ch 3 a deactivator?
Generally, activating groups are known for donating electrons. The OCH3 group, with its oxygen atom, is more electronegative than carbon, which leads to the oxygen atom pulling electrons away from the benzene ring through the inductive effect. This makes it an electron-withdrawing group, a property that classifies it as a deactivator.
To understand this further:
When a CH3 group is attached to a benzene ring, it acts as an electron-donating group through a mechanism called hyperconjugation. This effect involves the overlap of the C-H bonds of the CH3 group with the pi-electron system of the benzene ring. This overlap results in an increased electron density in the benzene ring, making it more reactive towards electrophilic attack.
In contrast, the OCH3 group, due to its oxygen’s higher electronegativity, draws electron density away from the benzene ring. This reduction in electron density makes the ring less susceptible to electrophilic attack, hence its classification as a deactivator.
Think of it like this:
An activating group makes the benzene ring more reactive, like adding fuel to a fire, making it easier for other molecules to react with it. A deactivating group, on the other hand, acts like a fire extinguisher, making the benzene ring less reactive and harder to interact with.
It’s important to remember that CH3 and OCH3 groups behave differently due to their distinct electronegativity and bonding characteristics.
Is CH3 a deactivating group?
Let’s break down why CH3 acts as an activating group. The CH3 group is electron-donating, meaning it pushes electron density towards the benzene ring. This increased electron density makes the ring more reactive towards electrophilic attack. Think of it like a magnet – the ring becomes more attractive to positively charged electrophiles.
The electron-donating nature of CH3 comes from its ability to donate electrons through a process called hyperconjugation. This involves the overlap of the filled sigma bonds of the CH3 group with the empty p-orbital of the benzene ring. This overlap creates a stabilizing effect, which makes the benzene ring more electron-rich.
In contrast, a deactivating group would be electron-withdrawing. These groups pull electron density away from the benzene ring, making it less reactive towards electrophiles. This is because the ring is now less electron-rich and therefore less attractive to positively charged species.
So, while CH3 boosts the rate of electrophilic aromatic substitution reactions by making the benzene ring more electron-rich, a deactivating group would hinder the reaction by making the ring less electron-rich.
Why do benzene ring -OCH3 and -CH3 react faster?
Here’s a breakdown of why they make the ring more reactive:
1. Electron Density Matters: The key to understanding why these groups make benzene react faster is electron density. The more electron-rich the benzene ring, the easier it is for electrophiles (electron-loving species) to attack. Think of it like a magnet attracting a piece of metal; the more magnetic the ring, the stronger the attraction.
2. Resonance and Hyperconjugation:
-OCH3: The oxygen in the -OCH3 group has lone pairs of electrons, and these electrons can participate in resonance with the pi system of the benzene ring. This pushes electron density towards the ortho and para positions of the benzene ring.
-CH3: The -CH3 group doesn’t have lone pairs, but it can engage in hyperconjugation. This involves the overlap of the C-H bonding electrons with the pi system of the benzene ring, again increasing electron density, mainly in the ortho and para positions.
3. The -I and +I Effects:
-OCH3: The -OCH3 group also has an inductive effect (-I). This effect pulls electron density away from the benzene ring, but the resonance effect is stronger in this case.
-CH3: The -CH3 group has a +I inductive effect, which pushes electron density towards the benzene ring.
Let’s Get Deeper:
The -OCH3 group is a bit more complex because it has both the resonance (+M) and inductive (-I) effects. The resonance effect, which is stronger, dominates, making the ring more electron-rich and reactive. However, the -I effect is still present and can influence the reactivity of the ring, depending on the specific reaction.
The -CH3 group only has the +I effect, making it a straightforward electron-donating group that increases electron density and reactivity. This means that the benzene ring with a -CH3 substituent will generally be more reactive towards electrophilic attack than the unsubstituted benzene ring.
In summary, the resonance effect of -OCH3 and the +I effect of -CH3 are the main contributors to the increased reactivity of the benzene ring. They both increase electron density in the ring, making it more susceptible to electrophilic attack.
See more new information: bmxracingthailand.com
Is Och3 Activating Or Deactivating: Understanding Electron Effects
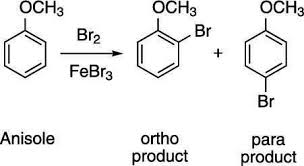
So, is OCH3 activating or deactivating? It’s activating!
Let’s break down why. You see, OCH3 is a methoxy group – think of it as a methyl group (CH3) linked to an oxygen atom (O). Now, this methoxy group is what we call an electron-donating group (EDG).
What’s an EDG?
Essentially, it’s a group that pushes electrons towards the aromatic ring, which is a special kind of cyclic system with alternating single and double bonds. This electron-donating effect makes the aromatic ring more reactive, hence the term activating.
Think of it like this: If you’re pushing more electrons towards the aromatic ring, it’s like making the ring more attractive to other molecules that might want to bond with it. And that’s what we mean by activating – it increases the ring’s reactivity.
How does OCH3 donate electrons?
Well, oxygen is more electronegative than carbon. This means oxygen pulls electrons towards itself, which can create a partial positive charge on the carbon atom in the methoxy group. But because the oxygen atom also has lone pairs of electrons, it can donate those electrons to the aromatic ring, creating a partial negative charge on the ring.
This shift of electrons makes the aromatic ring more reactive.
Here’s a visual breakdown:
OCH3 has a partial positive charge on the carbon atom because oxygen is pulling electrons towards itself.
OCH3 has a partial negative charge on the oxygen atom because it has lone pairs of electrons.
* The lone pairs of electrons on oxygen can donate into the aromatic ring, creating a partial negative charge on the ring.
This partial negative charge makes the aromatic ring more likely to undergo electrophilic aromatic substitution (EAS) reactions.
What’s EAS?
In simple terms, EAS is a reaction where an electrophile, which is a species that loves electrons, attacks the aromatic ring.
Think of it like this: An electrophile, which is electron-loving, is attracted to the partial negative charge on the aromatic ring. This attraction leads to a reaction where the electrophile attaches itself to the ring.
So, what does this mean for the OCH3 group’s position on the ring?
When you have an OCH3 group on an aromatic ring, it tends to direct incoming electrophiles to specific positions on the ring. It’s like the OCH3 group is guiding the electrophile to its favorite spot!
OCH3 is an ortho/para director. This means it directs incoming electrophiles to the ortho and para positions relative to itself.
Let’s clarify those terms:
Ortho means the position adjacent to the OCH3 group.
Para means the position opposite to the OCH3 group.
It’s like the OCH3 group is saying, “Hey, electrophile, come sit next to me or across from me, but don’t bother the other positions!”
Why is OCH3 ortho/para directing?
The OCH3 group’s electron-donating nature creates a higher electron density at the ortho and para positions. These positions become more attractive to electrophiles because they are more negatively charged.
Let’s summarize the key points:
OCH3 is an activating group because it donates electrons to the aromatic ring, increasing its reactivity.
OCH3 is an ortho/para director, meaning it directs incoming electrophiles to the ortho and para positions on the ring.
OCH3 is an electron-donating group due to the lone pairs of electrons on the oxygen atom.
Now, you have a solid understanding of why OCH3 is an activating group and how it influences the reactivity and substitution patterns of aromatic rings. This knowledge will be incredibly useful as you delve deeper into organic chemistry concepts.
FAQs:
Q1: Why is OCH3 considered an activating group?
A1: The OCH3 group is considered an activating group because it donates electrons to the aromatic ring, increasing its reactivity towards electrophiles. This electron donation arises from the lone pairs of electrons on the oxygen atom.
Q2: Why is OCH3 an ortho/para director?
A2:OCH3 is an ortho/para director because its electron donation creates a higher electron density at the ortho and para positions. This makes these positions more attractive to electrophiles, directing the substitution reaction to those specific positions.
Q3: How does the electron-donating effect of OCH3 differ from the electron-withdrawing effect of other groups?
A3:Electron-donating groups (EDGs) like OCH3 push electrons towards the aromatic ring, increasing its reactivity and making it more susceptible to electrophilic aromatic substitution. Conversely, electron-withdrawing groups (EWGs) pull electrons away from the ring, decreasing its reactivity and making it less likely to undergo EAS.
Q4: What are some other common activating groups?
A4: Other common activating groups include:
-NH2 (amino group)
-OH (hydroxyl group)
-CH3 (methyl group)
Q5: What are some examples of reactions where OCH3 plays a role?
A5:OCH3 plays a significant role in many reactions, including:
Friedel-Crafts alkylation
Friedel-Crafts acylation
Electrophilic aromatic substitution reactions
Q6: What are some common deactivating groups?
A6: Common deactivating groups include:
-NO2 (nitro group)
-COOH (carboxylic acid group)
-CN (cyano group)
Q7: What is the difference between an activating group and a deactivating group?
A7: Activating groups increase the aromatic ring’s reactivity towards electrophiles by donating electrons. Deactivating groups decrease the reactivity by withdrawing electrons.
Q8: How can I predict the products of reactions involving OCH3 and aromatic rings?
A8: To predict the products, consider:
OCH3 is an activating group and ortho/para directing.
* The incoming electrophile and its reactivity.
* The specific reaction conditions (e.g., catalyst, temperature).
Q9: Are there any exceptions to the ortho/para directing nature of OCH3?
A9: While OCH3 is generally an ortho/para director, there are situations where steric hindrance can influence the direction of attack.
Remember, organic chemistry is a complex and fascinating field. Understanding concepts like activating groups, deactivating groups, and directing effects is crucial for comprehending the behavior of organic molecules.
Activating and Deactivating Groups In Electrophilic
The more electron-rich the aromatic ring, the faster the reaction. Groups that can donate electron density to the ring make EAS reactions faster. If a substituent increases the rate of reaction relative to H it is called activating. If it decreases the rate relative to Master Organic Chemistry
Is OCH3 an activator or deactivator? – BYJU’S
Generally, the activating groups are electron-donating groups. As – OCH 3 has the oxygen atom which is more electronegative than the carbon atom so it can withdraw the electron BYJU’S
Ortho-, Para- and Meta- Directors in Electrophilic
Deactivating groups decrease the rate of electrophilic aromatic substitution, relative to hydrogen. If you look through the list of ortho- , para- directors, you might recognize that many of them are also Master Organic Chemistry
Why is -OCH3 more strongly activating than -CH3 in
Facts I know: 1) More the electron density in benzene ring, the faster the reaction. 2) Lone pair on -OCH3 group undergoes Chemistry Stack Exchange
16.1: Activation or Deactivation by Substituents on a Benzene Ring
The answer is that if you are trying to memorize such things, you are taking the wrong approach to organic chemistry. What you should be doing is trying to understand the Chemistry LibreTexts
7.4: Activation and Deactivation – Chemistry LibreTexts
Answer. In general, deactivating groups fall into two classes. Π-acceptors, such as carbonyls, if placed directly adjacent to the aromatic ring, slow down the reaction. Highly electronegative atoms, typically halogens, attached directly to the aromatic ring also slow down the reaction. libretexts.org
16.3: Directing Effects of Substituents in Conjugation with
As you saw in Section 16.4, a substituent on a benzene ring can be an activator or a deactivator. At the same time, a substituent can also be a meta director or an ortho/para Chemistry LibreTexts
AR4. Activation and Deactivation – Chemistry LibreTexts
Predict whether each of the following groups would be activating or deactivating towards electrophilic aromatic substitution. a) NH 2 b) CN c) OCH 3 d) Chemistry LibreTexts
16.4 Substituent Effects in Electrophilic Substitutions
What makes a group either activating or deactivating? The common characteristic of all activating groups is that they donate electrons to the ring, thereby making the ring more OpenStax
ortho–para-Directing Activators: –CH3, –OH, –&NH2,
TRANSCRIPT. 18.18: ortho – para -Directing Activators: –CH 3, –OH, –NH 2, –OCH 3. All ortho–para directors, excluding halogens, are activating groups. These groups donate JoVE
Ortho Meta Para Directors – Activating And Deactivating Groups
Easy Way To Determine Ortho-Para Or Meta Directing Eas Without Memorizing Anything!
Trick For Determining Edg Or Ewg
Multiple Substituents | Aromatic Compounds | Organic Chemistry | Khan Academy
Activation / Deactivation Of Aromatic Rings
Ortho/Meta/Para Directors
18.3 Eas Ortho-Para Directors Vs Eas Meta Directors | Organic Chemistry
Why Does No2 Group Show Its Effect Only At Ortho- And Para- Positions And Not At Meta- Position?
Aromatic, Antiaromatic, Or Nonaromatic – Huckel’S Rule – 4N+2 – Heterocycles
Acids \U0026 Bases – Inductive Effect, Electronegativity, Hybridization, Resonance \U0026 Atomic Size
Link to this article: is och3 activating or deactivating.
See more articles in the same category here: bmxracingthailand.com/what
