What can Raney nickel reduce?
Let’s break down these types of compounds and see how Raney nickel can help.
Alkynes and Alkenes: Raney nickel readily reduces alkynes (compounds with a triple bond) to alkenes (compounds with a double bond) and then further to alkanes (compounds with only single bonds). This ability to selectively reduce these bonds makes it a valuable tool for organic synthesis.
Nitriles: Nitriles are compounds with a carbon triple-bonded to a nitrogen. Raney nickel reduces them to amines. This reaction is often used in the production of pharmaceuticals and other chemicals.
Dienes and Aromatics:Dienes are compounds with two double bonds. Raney nickel can be used to selectively reduce one or both of these double bonds. Aromatic compounds are characterized by a ring of carbons with alternating single and double bonds. Raney nickel can be used to reduce these double bonds, resulting in the formation of non-aromatic compounds.
Carbonyl-containing compounds: Carbonyl compounds have a carbon double-bonded to an oxygen atom. Raney nickel can reduce these compounds to alcohols. This is particularly useful in the production of alcohols from aldehydes and ketones.
Heteroatom-heteroatom bonds: These bonds are between two atoms that aren’t carbon. Raney nickel can break these bonds, which can be useful in the synthesis of various compounds. For example, it can be used to reduce hydrazines to amines and nitro groups to amines.
The power of Raney nickel lies in its ability to selectively reduce different types of bonds in a molecule, making it a versatile tool for organic chemists.
Can B2H6 reduce carboxylic acid?
Diborane is a powerful reducing agent, but it’s not the first choice for carboxylic acids. Typically, you’d use lithium aluminum hydride (LiAlH4) to convert carboxylic acids to alcohols.
Now, why is diborane often overlooked for this job? Well, it’s because diborane is a bit more finicky when it comes to carboxylic acids. It can react with them, but it’s not as efficient or reliable as lithium aluminum hydride. Lithium aluminum hydride is a stronger reducing agent and can handle the task with more ease.
Think of it like this: diborane is like a gentle nudge, while lithium aluminum hydride is like a full-on push. Both can get the job done, but one is clearly more forceful and effective.
Let’s dive a bit deeper into why lithium aluminum hydride is the go-to for carboxylic acid reductions. It’s because lithium aluminum hydride is a complex metal hydride that can readily donate hydride ions (H–). These hydride ions are the key players in the reduction process, attacking the carbonyl group of the carboxylic acid and ultimately replacing it with a hydroxyl group, forming an alcohol.
Diborane, on the other hand, is a weaker reducing agent. While it can react with carboxylic acids, it often leads to side reactions and less predictable outcomes. It’s less efficient at donating hydride ions, making it a less reliable choice for this specific transformation.
So, in a nutshell, diborane can be used to reduce carboxylic acids to alcohols, but lithium aluminum hydride is the preferred choice due to its greater effectiveness and reliability. It’s a more powerful and efficient reducing agent, making it the go-to option for achieving the desired transformation.
Can catalytic hydrogenation reduce carboxylic acid?
High temperature and pressure are key. Think of it like this: you need to give the reaction a good push to get it going. The hydrogen gas needs to be able to break the strong bond in the carboxylic acid molecule, and that requires some extra energy.
Catalysts are also essential. They’re like the cheerleaders of the chemical world, encouraging the reaction to happen faster and more efficiently. Common catalysts used in catalytic hydrogenation of carboxylic acids include palladium, platinum, and nickel.
Let’s dive deeper into how this process works:
Understanding the Chemistry:
The reduction of a carboxylic acid to an alcohol involves the addition of two hydrogen atoms. This reaction is often referred to as “hydrogenolysis”, where the carbon-oxygen bond in the carboxylic acid group is broken.
Here’s a breakdown of what happens:
1. Activation: The catalyst, typically a metal like palladium, platinum, or nickel, helps activate the hydrogen gas. This means the hydrogen molecule breaks into two hydrogen atoms, which are highly reactive.
2. Adsorption: The carboxylic acid molecule and the hydrogen atoms attach to the surface of the catalyst.
3. Reaction: The hydrogen atoms react with the carboxylic acid molecule, breaking the carbon-oxygen bond and forming an alcohol.
4. Desorption: The newly formed alcohol and the catalyst detach from each other, allowing the catalyst to repeat the process.
Practical Applications:
This reaction is important for a lot of different things in the chemistry world. It’s used in the production of various alcohols, which are essential building blocks for many products, such as pharmaceuticals, plastics, and cosmetics.
For example, the catalytic hydrogenation of benzoic acid produces benzyl alcohol, a key ingredient in many perfumes.
It’s also worth noting that this reaction can be quite selective. For example, if you have a molecule with multiple functional groups, you can carefully choose the catalyst and reaction conditions to target just the carboxylic acid group. This allows chemists to control which parts of a molecule are modified, which is crucial for synthesizing specific compounds.
Can carboxylic acid be reduced by H2 nickel?
Hydrogen with Raney nickel is a powerful reducing agent. It can tackle a lot of unsaturated compounds, like alkenes and alkynes. However, it generally doesn’t touch carboxylic acids. That’s where sodium borohydride comes in. Sodium borohydride is a more selective reducing agent, often used in the presence of methanol. It targets carbonyls like aldehydes and ketones, but leaves esters, amides, and carboxylic acids alone.
So, can you reduce carboxylic acids with hydrogen and Raney nickel? The short answer is no.
Here’s why:
Carboxylic acids are pretty stable. They have a strong carbon-oxygen double bond (C=O) and an oxygen atom bonded to a hydrogen atom (O-H). This structure makes it harder to break the bonds and add hydrogen atoms to the molecule.
Think of it like this: A carboxylic acid is a bit like a stubborn castle. Hydrogen and Raney nickel are like a friendly but not very strong army. They can conquer easy targets, like alkenes and alkynes, but they can’t break through the walls of a fortified carboxylic acid.
To conquer this stubborn fortress, you need stronger weapons, like lithium aluminum hydride (LiAlH4). This is a much more powerful reducing agent than hydrogen and Raney nickel, and it can successfully break through the carboxylic acid’s defenses to add hydrogen atoms and form an alcohol.
So, while hydrogen and Raney nickel are great for tackling unsaturated compounds, they’re not the best tools for tackling carboxylic acids. You’ll need a more powerful reducing agent like lithium aluminum hydride for that task.
What are the limitations of Raney nickel?
One key limitation is lack of chemoselectivity. This means that Raney nickel can sometimes react with other functional groups in a molecule, leading to unwanted side reactions. For example, if you’re trying to break a carbon-selenium bond in a molecule that also contains a carbon-oxygen bond, Raney nickel might react with both, making it difficult to get the desired product.
Another important consideration is that Raney nickel is pyrophoric, meaning it can ignite spontaneously in air. This requires careful handling and storage, often in a sealed container under an inert atmosphere like nitrogen.
Let’s dive a bit deeper into the lack of chemoselectivity. Imagine you have a molecule with a carbon-selenium bond and a carbon-oxygen bond. You want to break only the carbon-selenium bond, but Raney nickel might not be the best choice. It’s like trying to pick a specific apple from a basket full of different fruits – you might end up grabbing a pear instead!
To overcome this challenge, chemists often use other techniques like protecting groups. These groups can temporarily block certain functional groups on a molecule, preventing unwanted reactions.
For example, if you want to break the carbon-selenium bond without affecting the carbon-oxygen bond, you might add a protecting group to the carbon-oxygen bond before using Raney nickel. This prevents the nickel from reacting with the oxygen. Once the carbon-selenium bond is broken, you can remove the protecting group, leaving you with the desired product.
While Raney nickel is a powerful tool, its pyrophoric nature and lack of chemoselectivity require careful consideration and planning. It’s not always the best choice for every reaction. By understanding these limitations, you can make informed decisions about whether Raney nickel is the right reagent for your specific needs.
Which reagent cannot reduce carboxylic acid?
The carbonyl carbon in esters, amides, and carboxylic acids is connected to an atom with a lone pair that participates in resonance with the carbonyl group’s double bond. This resonance makes the carbonyl group less electrophilic and harder to reduce. NaBH4, being a weaker reducing agent, simply doesn’t have the power to overcome this resonance stabilization and reduce these functional groups.
Think of it like this: Imagine you’re trying to push a heavy rock uphill. NaBH4 is like a small, weak person trying to push the rock. It just doesn’t have enough strength. Now, imagine a powerful bulldozer trying to move the same rock. That’s like a stronger reducing agent like LiAlH4; it has the power to overcome the resistance and get the job done.
LiAlH4, a more potent reducing agent, can tackle the tougher task of reducing carboxylic acids. It’s like the bulldozer; it has the necessary power to break through the resonance stabilization and reduce the carbonyl group.
Here’s a deeper look at why NaBH4 is less effective than LiAlH4:
Hydride Reactivity: NaBH4 has a less reactive hydride ion compared to LiAlH4. This difference in reactivity stems from the electronegativity of the metal cation. Aluminum is less electronegative than boron, making the hydride ion in LiAlH4 more nucleophilic and reactive.
Steric Hindrance:NaBH4 is also less bulky than LiAlH4. This smaller size makes it less effective at attacking sterically hindered carbonyl groups. Carboxylic acids often have bulky substituents surrounding the carbonyl group, further hindering the attack of NaBH4.
Solvent Compatibility: NaBH4 is typically used in protic solvents like methanol or ethanol, whereas LiAlH4 requires anhydrous conditions. Protic solvents can deactivate NaBH4 by reacting with its hydride ions, further reducing its effectiveness.
In summary, while NaBH4 is a great tool for reducing aldehydes and ketones, it lacks the punch to overcome the resonance stabilization present in carboxylic acids. LiAlH4, on the other hand, is powerful enough to accomplish this reduction.
Why can’t NaBH4 reduce carboxylic acids?
Sodium borohydride is great at reducing aldehydes and ketones to alcohols, but it struggles with carboxylic acids. The reason lies in the reactivity of the carbonyl group in carboxylic acids. This group is more stable than the carbonyl group in aldehydes or ketones. In simpler terms, the carbon in the carbonyl group of carboxylic acids is less reactive than the one in aldehydes or ketones. This difference in reactivity means that sodium borohydride lacks the power to overcome the stronger bond in carboxylic acids.
Think of it this way: Imagine you have a strong lock and a weak key. The key may be able to open other, less secure locks, but it won’t be able to open the strong lock. In the same way, sodium borohydride can “open” the less secure carbonyl groups in aldehydes and ketones, but it’s not strong enough to “open” the more secure carbonyl group in carboxylic acids.
What happens when NaBH4 reacts with a carboxylic acid?
When sodium borohydride reacts with a carboxylic acid, an aldehyde is produced as an intermediate. This aldehyde is very reactive and quickly reacts further, forming back into the original carboxylic acid. This means that the aldehyde can’t be isolated.
How can you reduce carboxylic acids?
If you want to reduce carboxylic acids to alcohols, you need a stronger reducing agent like lithium aluminum hydride (LiAlH4). This powerful reagent can overcome the stability of the carbonyl group in carboxylic acids and successfully reduce them to alcohols.
What dissolves carboxylic acid?
Let’s dive a little deeper into why these solvents work so well with carboxylic acids.
Ethanol is a good solvent for carboxylic acids because it can form hydrogen bonds with the carboxyl group (-COOH) of the acid. Ethanol has a hydroxyl group (-OH) that can participate in hydrogen bonding, making it a good choice for dissolving carboxylic acids.
Toluene is a good solvent for carboxylic acids because it is a nonpolar solvent. This means that it can dissolve nonpolar compounds like carboxylic acids. While toluene doesn’t form hydrogen bonds with the carboxyl group, it can still interact with the hydrophobic (water-repelling) part of the molecule.
Diethyl ether is a good solvent for carboxylic acids because it is a polar solvent. It has a dipole moment, which allows it to interact with the polar carboxyl group of the acid. This interaction is similar to how water dissolves salts – a polar solvent like water interacts with the ions of salt to pull them apart.
Think of it like this: ethanol, toluene, and diethyl ether are all like keys that unlock the “door” to dissolve the carboxylic acid. They each use different mechanisms, but they all get the job done.
Does Raney nickel reduce ketones?
Raney nickel is a highly porous nickel-aluminum alloy that is a fantastic catalyst for various chemical reactions. One of its most prominent uses is in the reduction of ketones to secondary alcohols.
This method is incredibly efficient when using refluxing 2-propanol containing a trace of HCl. The refluxing 2-propanol provides a suitable solvent environment, while the trace of HCl acts as a promoter, enhancing the catalytic activity of the Raney nickel.
Let’s break down the process:
Ketone Reduction: When a ketone is exposed to Raney nickel in this specific reaction environment, the nickel catalyst facilitates the transfer of hydrogen atoms from the 2-propanol to the ketone. This hydrogenation process results in the conversion of the ketone group (C=O) to a secondary alcohol group (C-OH).
2-Propanol as a Hydrogen Source: The 2-propanol acts as a hydrogen source, supplying the necessary hydrogen atoms for the reduction. The refluxing conditions ensure that the 2-propanol is constantly vaporizing and condensing, providing a continuous supply of hydrogen.
HCl as a Promoter: The addition of a trace of HCl plays a crucial role in enhancing the catalytic activity of the Raney nickel. This small amount of acid helps activate the nickel catalyst, making it more efficient in promoting the hydrogenation reaction.
In summary, the combination of Raney nickel, refluxing 2-propanol, and a trace of HCl creates a highly effective system for reducing ketones to secondary alcohols. This method is widely employed in organic synthesis due to its reliability and efficiency.
Let’s delve a bit deeper into the benefits of using Raney nickel for this specific reaction:
High Activity:Raney nickel boasts an incredibly high surface area, making it a highly active catalyst. This characteristic allows for rapid and efficient hydrogenation of ketones.
Selectivity:Raney nickel exhibits excellent selectivity for reducing ketones, meaning it preferentially targets the ketone group without significantly affecting other functional groups within the molecule.
Mild Conditions: The reaction conditions are relatively mild, requiring only refluxing 2-propanol and a trace of HCl. This makes the process suitable for a wide range of organic molecules.
Ease of Handling:Raney nickel is commercially available and relatively easy to handle, making it a convenient and practical catalyst for laboratory and industrial applications.
In conclusion, Raney nickel offers a versatile and effective solution for reducing ketones to secondary alcohols. Its high activity, selectivity, mild reaction conditions, and ease of use make it a valuable tool for organic chemists.
Can LiAlH4 reduce carboxylic acids?
Let’s take a look at how LiAlH4 does this. LiAlH4 reacts with carboxylic acids to form an alkoxide intermediate. This intermediate is then protonated by water, yielding the corresponding primary alcohol. This reaction typically requires a large excess of LiAlH4 and is usually conducted in an ethereal solvent, such as diethyl ether or tetrahydrofuran (THF).
Here’s an example:
The reduction of acetic acid to ethanol by LiAlH4:
CH3COOH + 4[H] → CH3CH2OH + H2O
LiAlH4 is a very strong reducing agent and can reduce a wide variety of functional groups, including carboxylic acids, esters, and acid halides. It’s important to note that LiAlH4 is extremely reactive with water and will react violently if exposed to air. Therefore, it’s crucial to use LiAlH4 under anhydrous conditions.
LiAlH4 is a powerful reagent that can reduce carboxylic acids to their corresponding primary alcohols, opening up a world of synthetic possibilities. It’s a valuable tool for organic chemists and a testament to the power of chemical reduction.
See more here: Can B2H6 Reduce Carboxylic Acid? | Does Raney Nickel Reduce Carboxylic Acids
What is Raney nickel catalyzed hydrogenation?
Raney nickel is a highly porous nickel-aluminum alloy that’s a powerful catalyst for hydrogenation reactions. It’s commonly used to add hydrogen atoms to unsaturated compounds, like carboxylic acids.
This process, called Raney nickel catalyzed hydrogenation, is a green and efficient way to reduce these acids. What makes it so special?
Firstly, it’s incredibly selective. This means it can target specific bonds in a molecule without affecting others. This is a big advantage when working with complex molecules, ensuring you get the desired product.
Secondly, this method is mild. This means it can be done at relatively low temperatures and pressures, making it safer and easier to control.
Finally, it’s environmentally friendly. By using water as the solvent, it avoids the use of harmful organic solvents, reducing waste and minimizing environmental impact.
So, how does it work? Well, Raney nickel acts as a catalyst, providing a surface for the hydrogen gas to attach to. This activated hydrogen can then react with the unsaturated bonds in the carboxylic acid, transforming them into saturated compounds.
A Safe and Practical Method
The sodium borohydride-Raney nickel (W6) system is a particularly effective way to perform this reaction. Sodium borohydride is a reducing agent, providing the hydrogen needed for the reaction. The Raney nickel acts as a catalyst, speeding up the process. This combination is especially useful for reducing unsaturated carboxylic acids because it’s highly selective and can be used in water, making it a safe and practical method for various applications.
The use of sodium borohydride in this system is particularly advantageous. It’s a powerful reducing agent, capable of reducing a wide range of functional groups, including carbonyl groups and nitro groups. However, it’s also very selective, only reacting with the desired groups in most cases.
Combining this selectivity with the advantages of Raney nickel catalysis, we get a powerful tool for organic chemists. It’s a method that’s both effective and environmentally conscious, leading to a cleaner and safer approach to chemical synthesis.
Does CO inhibit the catalytic properties of Raney® nickel?
Let me break down what’s happening here. Transfer hydrogenation is a chemical reaction where hydrogen atoms are transferred from one molecule to another. In this case, the hydrogen comes from a source called a hydrogen donor, and it’s added to the acetophenone molecule. Raney® nickel acts as a catalyst, speeding up this reaction.
Now, CO is known to bind to metal surfaces, and Raney® nickel is a metal. This binding can block the active sites on the nickel surface where the hydrogenation reaction takes place. In simpler terms, CO can get in the way and prevent the nickel from doing its job effectively.
Our experiment was designed to see if this was happening. By comparing the hydrogenation results with and without CO, we could see if CO was indeed inhibiting the catalytic activity of Raney® nickel.
Figures 1 and 8 would show us the extent of the reaction (how much acetophenone was converted) under both conditions. If the reaction proceeded much slower with CO, that would confirm that CO is inhibiting the catalytic properties of Raney® nickel.
What is Raney nickel used for?
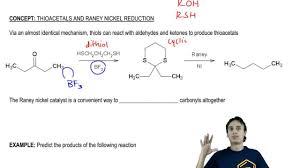
Let’s delve deeper into this specific application of Raney nickel. The reduction of C-S bonds to C-H bonds is a two-step process. First, the ketone is converted to a thioketal. This is done by reacting the ketone with a thiol, such as ethanethiol. The second step is the reduction of the thioketal to an alkane. This is done by using Raney nickel as the catalyst and hydrogen gas as the reducing agent. This reaction is also known as the desulfurization of a thioketal.
This process has several advantages over the traditional Wolff-Kishner reaction, a common method used to convert ketones to alkanes. Firstly, the reaction conditions for the Raney nickel-catalyzed desulfurization are milder than those for the Wolff-Kishner reaction. Secondly, the Raney nickel-catalyzed desulfurization is more efficient than the Wolff-Kishner reaction. This means that the reaction proceeds faster and produces more of the desired product. Finally, the Raney nickel-catalyzed desulfurization is more selective than the Wolff-Kishner reaction. This means that the reaction is less likely to produce unwanted side products.
Overall, Raney nickel-catalyzed desulfurization is a very useful and versatile method for converting ketones to alkanes. It is a mild, efficient, and selective method that can be used in a variety of applications.
See more new information: bmxracingthailand.com
Does Raney Nickel Reduce Carboxylic Acids?
Hey there, chemistry enthusiasts! We’re diving into the exciting world of organic chemistry today, specifically exploring the question: does Raney nickel reduce carboxylic acids? This is a topic that comes up often, and it’s one that we’ll dissect in detail to get a clear understanding.
First things first, Raney nickel is a solid catalyst that’s super useful in hydrogenation reactions. It’s a finely divided nickel-aluminum alloy with a porous structure that offers a large surface area for chemical reactions. This makes it a champion in facilitating the addition of hydrogen to various organic molecules.
Now, let’s get to the juicy part – carboxylic acids! These are organic compounds characterized by the presence of a carboxyl group (-COOH). They’re found in all sorts of things, from vinegar (acetic acid) to your favorite fatty acids in your food.
The main question is, can Raney nickel be used to reduce carboxylic acids to aldehydes or alcohols? Well, the answer is a little more complicated than a simple yes or no.
Here’s the breakdown:
Generally speaking, Raney nickel is not the go-to catalyst for reducing carboxylic acids directly to aldehydes or alcohols. While it can be used to hydrogenate some carbonyl compounds, it’s not as effective when it comes to carboxylic acids. This is because the carboxyl group is a bit more stubborn and less prone to reduction compared to other carbonyl groups.
The challenge lies in the stability of the carboxyl group. It’s a pretty strong group, and it takes a lot of energy to break the bond between the carbon and oxygen atoms.
However, there are ways to make it work! We can still achieve this reduction, but it usually requires more drastic conditions or alternative catalysts.
Let’s explore some common methods used to reduce carboxylic acids:
1. Lithium Aluminum Hydride (LiAlH4): This is a powerful reducing agent that can handle the reduction of carboxylic acids to primary alcohols. It works by transferring hydride ions to the carboxyl group, breaking the carbon-oxygen bond and replacing it with a hydrogen. However, LiAlH4 is a very reactive reagent and requires careful handling in anhydrous conditions.
2. Sodium Borohydride (NaBH4): While not as powerful as LiAlH4, NaBH4 can reduce certain carboxylic acids, specifically those with electron-withdrawing groups attached. It’s a more convenient reagent to work with than LiAlH4, but it’s important to note that it’s not always effective for reducing carboxylic acids.
3. Catalytic Hydrogenation with Modifiers: We can enhance the effectiveness of Raney nickel for carboxylic acid reduction by employing modifiers. These modifiers can influence the selectivity and reactivity of the catalyst. Common examples include adding a metal like platinum or palladium to the Raney nickel surface.
4. Other Catalytic Methods: Other catalytic systems, like Ruthenium-based catalysts, have also shown success in reducing carboxylic acids to aldehydes or alcohols under specific conditions.
It’s crucial to understand that the choice of method depends on various factors, including the specific carboxylic acid you’re working with, the desired product, and the desired reaction conditions.
So, to summarize, while Raney nickel may not be the perfect fit for directly reducing carboxylic acids to aldehydes or alcohols, we can definitely achieve this reduction with alternative methods. We have powerful reducing agents like LiAlH4 and NaBH4, along with the option of modifying Raney nickel or employing other catalysts. It’s all about finding the right approach for the specific reaction you’re aiming for!
FAQs: Does Raney Nickel Reduce Carboxylic Acids?
Q: What are some examples of carboxylic acids that Raney nickel can’t reduce?
A: Raney nickel is generally not effective for reducing aromatic carboxylic acids like benzoic acid, or saturated carboxylic acids like acetic acid. It’s better suited for reducing more reactive carbonyl compounds.
Q: What are some of the limitations of using LiAlH4 for carboxylic acid reduction?
A: LiAlH4 is a powerful reducing agent, but it’s also quite dangerous. It reacts violently with water and requires careful handling under anhydrous conditions. It’s important to have proper training and safety precautions in place when working with LiAlH4.
Q: What are some factors that affect the efficiency of catalytic hydrogenation for carboxylic acid reduction?
A: Several factors can influence the efficiency of catalytic hydrogenation, including:
The nature of the catalyst: The choice of catalyst, its surface area, and its activity can all affect the reaction rate and selectivity.
The nature of the carboxylic acid: Different carboxylic acids have different reactivity towards reduction.
The reaction conditions: The temperature, pressure, and solvent used can all impact the efficiency of the reaction.
Q: Can you provide an example of a reaction where Raney nickel is used to reduce a carboxylic acid?
A: While Raney nickel is not typically used for reducing carboxylic acids directly to aldehydes or alcohols, it can be used for certain reactions involving carboxylic acid derivatives. For example, it can be used to reduce esters to alcohols, where the ester undergoes hydrogenolysis and cleaves the ester bond.
Q: Are there any other ways to reduce carboxylic acids besides the methods mentioned?
A: Yes, there are other methods! One promising approach is electrochemical reduction. It involves applying an electric current to a solution of the carboxylic acid, which can facilitate the reduction to an aldehyde or alcohol. This method has gained attention as a more environmentally friendly alternative to conventional methods.
Q: Is there a way to increase the reactivity of Raney nickel to reduce carboxylic acids?
A: While Raney nickel is not the primary catalyst for reducing carboxylic acids, its reactivity can be enhanced through various techniques:
Pre-treatment: Treatment of the Raney nickel catalyst with specific chemicals or conditions can activate its surface and improve its performance.
Alloying: Adding other metals to the nickel alloy can alter its catalytic properties and enhance its effectiveness for specific reactions.
Support Materials: Using a support material like carbon or alumina can increase the surface area of the catalyst and improve its stability.
Q: What are some applications of reducing carboxylic acids to aldehydes or alcohols?
A: Reducing carboxylic acids is an important step in the synthesis of various organic compounds, including:
Pharmaceuticals: Many pharmaceuticals contain alcohol or aldehyde functional groups that are often prepared through the reduction of carboxylic acids.
Fragrances and flavors: Aldehydes and alcohols are commonly used as fragrances and flavors in various consumer products.
Polymers: Reducing carboxylic acids can be a key step in the synthesis of polymers and other materials.
Q: What are some safety precautions to take when working with reducing agents like LiAlH4?
A: Always wear appropriate personal protective equipment (PPE) when working with reducing agents. This includes gloves, a lab coat, and safety glasses. It’s essential to work in a well-ventilated area and avoid contact with water, as LiAlH4 reacts violently with it. Always follow the manufacturer’s safety guidelines and store the reagent in a safe and secure location.
Remember, always consult reliable chemistry resources and lab manuals for detailed information and proper safety protocols when working with reducing agents and catalysts. Stay curious and keep exploring the exciting world of chemistry!
Can hydrogen on platinum reduce carboxylic acids and esters?
Can $\ce{H2/Pt}$ reduce carboxylic acids and esters? I am confused about the reducing nature of hydrogen in presence of platinum. In some books, it reduces carboxylic acid and ester while in others, not. I have checked Finar and Boyd books for it. Chemistry Stack Exchange
Reagent Friday: Raney Nickel – Master Organic Chemistry
Reduction Of Sulfur Groups (Dithianes) To Alkanes With Raney Nickel What it’s used for: Like palladium on carbon (Pd/C) and platinum on carbon (Pt/C), Raney nickel can be used for the Master Organic Chemistry
Raney® nickel-catalyzed hydrodeoxygenation and
Raney® nickel is recognized as a widely-used industrial hydrogenation catalyst for the reduction of monosaccharide sugars, nitriles, nitro- and carbonyl ScienceDirect
Myers Reduction Chem 115 – Harvard University
Borane is commonly used for the reduction of carboxylic acids in the presence of esters, lactones, amides, halides and other functional groups. In addition, borane rapidly Harvard Web Publishing
Raney Nickel–Catalyzed Hydrogenation of Unsaturated
A mild, selective, and green method for the reduction of unsaturated carboxylic acids with sodium borohydride–Raney nickel (W6) system in water is Taylor & Francis Online
21.7: Reduction of Carbonyl Compounds and Acid
Another way to reduce carbonyl groups and acid chlorides is through the catalytic addition of hydrogen. Just like the C=C bond, the C=O bond is capable of adding one mole of hydrogen. The catalyst Chemistry LibreTexts
Some like it weak: different activity of Raney® nickel in
However, the most applicable catalyst of the family is certainly Raney® nickel (metallic nickel sponge, skeletal nickel), primarily in conventional hydrogenation ScienceDirect
General synthesis of primary amines via reductive
Here a nickel catalyst—formed by pyrolysis of a nickel complex on a γ-Al2O3 support—is shown to be highly active for synthesis of primary amines via Nature
Raney Nickel Catalyzed Hydrogenation of Unsaturated
practical method for the reduction of unsaturated carboxylic acids in water. We have selected sodium borohydride as hydrogen source and Raney nickel (W6 grade) as ResearchGate
Nabh4, Lialh4, Dibal Reduction Mechanism, Carboxylic Acid, Acid Chloride, Ester, \U0026 Ketones
Raney Nickel + Clemmensen Reductions And Kmno4 Oxidation Of Benzylic Carbons
Reducing Carboxylic Acids To Alcohols
Lithium Aluminum Hydride Reduction Of Carboxylic Acids (Mechanism)
Reduction Of Carbonyls To Alcohols
Raney Nickel Reduction
Raney Nickel (Ni-H)
Reduction Of Carboxylic Acids | Carboxylic Acids And Derivatives | Organic Chemistry | Khan Academy
Link to this article: does raney nickel reduce carboxylic acids.
See more articles in the same category here: https://bmxracingthailand.com/what
