What is the zymogen of a proenzyme?
Think of it like this: A zymogen is like a puzzle with a missing piece. It’s ready to function but needs that extra piece to become complete. This missing piece is a specific portion of the protein that needs to be removed. This removal can happen through a chemical process, like using an acid, or by another enzyme that snips off the right piece. Once this happens, the zymogen becomes active and can do its job!
This process of activation is crucial for many reasons. For one thing, it allows cells to control when and where enzymes are active. It’s like having a switch that turns the enzyme on and off when needed. This is important because enzymes can be powerful catalysts, and if they were always active, they could cause damage. Another reason is that zymogens are often produced in inactive forms because they might be harmful to the cells that produce them. This is especially true for digestive enzymes, which break down food. They need to be inactive until they reach the digestive system, where they are needed to break down food.
Here are some examples of zymogens and their active forms:
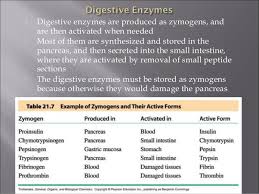
Pepsinogen (zymogen) is activated to Pepsin (active enzyme) in the stomach to break down proteins.
Trypsinogen (zymogen) is activated to Trypsin (active enzyme) in the small intestine to break down proteins.
Chymotrypsinogen (zymogen) is activated to Chymotrypsin (active enzyme) in the small intestine to break down proteins.
These are just a few examples of the many zymogens that are involved in various cellular processes. They play a vital role in maintaining cellular function and ensuring that essential processes like digestion occur efficiently and without harm.
What is the difference between zymogen and pepsinogen?
Pepsinogen is the inactive precursor of pepsin. This means that pepsinogen is a form of pepsin that’s not yet ready to do its job. Imagine it like a “locked and loaded” weapon that needs a trigger to fire.
Think of pepsin as the main proteolytic enzyme in our stomach juice. Proteolytic enzymes are like tiny scissors that break down proteins in our food. Pepsinogen is the “locked and loaded” version of these scissors, while pepsin is the “unlocked” and ready-to-cut version.
But how does pepsinogen turn into pepsin? The answer lies in the stomach’s acidic environment. When pepsinogen enters the stomach, the acidic conditions “unlock” it. This transformation from pepsinogen to pepsin is essential for the digestion of proteins in our diet.
Here’s a simple analogy:
* Imagine pepsinogen as a puzzle with a missing piece.
* The acidic environment in the stomach provides that missing piece, allowing the puzzle to be completed and become active as pepsin.
This activation process ensures that pepsin, a powerful enzyme, is only active within the stomach, where it’s needed for digestion. This prevents it from causing damage in other parts of the body.
The discovery of pepsinogen is quite interesting. It was first isolated and crystallized from the gastric mucosa of swine (pigs). Since then, several types of pepsinogen have been identified. This means that there isn’t just one “flavor” of pepsinogen; different variations exist, each with its own unique properties.
What is the difference between zymogen and Isozyme?
Isozymes are multiple forms of an enzyme that catalyze the same reaction. They can have different amino acid sequences, but they still perform the same function. Think of them like different versions of the same software – they might look slightly different, but they achieve the same outcome.
Zymogens are inactive precursors of enzymes. These precursors need to be activated before they can perform their catalytic function. Imagine a puzzle – a zymogen is like the pieces in a box, while the active enzyme is the completed puzzle. A specific change, like a small piece being added or removed, is needed to transform the inactive zymogen into the active enzyme.
Think of isozymes as different chefs who can make the same dish. Each chef might use different ingredients or techniques, but they all produce the same delicious meal. In contrast, zymogens are like raw ingredients that need to be cooked before they become a tasty dish.
Now, let’s dive deeper into what makes isozymes unique:
Different Structure: Each isozyme can have a slightly different amino acid sequence, giving them variations in their structure.
Tissue Specificity:Isozymes can be expressed in different tissues or at different developmental stages. This allows for tissue-specific regulation of enzyme activity.
Kinetic Properties:Isozymes may have different kinetic properties, meaning they might work at different speeds or under different conditions. For example, they might be more sensitive to certain temperatures or pH levels.
Regulation: The expression and activity of isozymes can be regulated differently, providing fine-tuned control over metabolic processes.
These differences are important because they allow organisms to adapt to changing environments and fine-tune their metabolism.
What is the difference between apoenzyme and proenzyme?
An apoenzyme is like a puzzle piece – it’s an inactive enzyme that needs a cofactor to complete its structure and function. Think of the cofactor as the missing piece that allows the apoenzyme to become a fully functional holoenzyme. Cofactors can be metals, like zinc or magnesium, or organic molecules, like vitamins. Once the cofactor is in place, the apoenzyme transforms into a powerful, active enzyme ready to do its job.
Proenzymes, or zymogens, are a bit different. Imagine a chef prepping a dish – they start with an inactive ingredient, which is then transformed into the final, delicious dish. In the same way, zymogens are inactive forms of enzymes that need a biochemical transformation to become active. This transformation often involves hydrolysis, which is the breaking down of a molecule using water. This process “activates” the zymogen, giving it the power to perform its specific enzymatic task.
Think of pepsinogen, the inactive form of the digestive enzyme pepsin. In the stomach, pepsinogen undergoes hydrolysis, transforming into pepsin, the active enzyme that helps break down proteins in our food.
Here’s a simple way to remember the difference:
Apoenzyme: Think “apo” like “apart,” signifying an incomplete enzyme needing a cofactor.
Proenzyme: Think “pro” like “before,” signifying an inactive form that needs transformation to become active.
Understanding the difference between apoenzymes and proenzymes is essential for grasping the intricacies of enzyme function and regulation in biological systems. These processes are fundamental to life, ensuring that our bodies can break down nutrients, synthesize molecules, and maintain a delicate balance.
What is the proenzyme?
A proenzyme is an inactive form of an enzyme. Think of it like a sleeping giant – it has all the potential to be powerful, but it needs a little nudge to get going.
So, how do these proenzymes wake up? They undergo a biochemical change, like a chemical reaction. This change can involve several things:
Hydrolysis: This is like cutting the puzzle piece in the right place to reveal the hidden part.
Configuration Change: Sometimes, a proenzyme needs to fold into the right shape to become active. It’s like changing the puzzle piece’s orientation.
Once this change happens, the proenzyme becomes a fully functioning enzyme, ready to carry out its specific task.
The Importance of Proenzymes
Proenzymes are really important because they help our bodies control when and where enzymes are active. It’s like having a safety switch that prevents enzymes from going wild and causing damage.
Here’s a quick example:
Pepsinogen is the proenzyme form of pepsin, a powerful enzyme that digests proteins in our stomach.
* When food enters our stomach, pepsinogen is activated to pepsin. This ensures that protein digestion only happens when and where it’s needed, preventing damage to our stomach lining.
Proenzymes are found all over our bodies, playing key roles in various processes, from digestion to blood clotting. They’re a testament to the amazing complexity and control that exists within our biological systems.
What is a zymogen?
A zymogen is essentially an inactive form of an enzyme. Think of it as a sleeping giant, just waiting for the right signal to wake up and do its job. These precursors are created and secreted by living cells, and they need a bit of activation before they can get to work.
How do zymogens get activated? They’re often transformed into their active form by a process called proteolytic cleavage. This means a specific part of the zymogen molecule is snipped off, like removing a safety pin, allowing it to fold into the proper shape and become a fully functional enzyme.
Why are zymogens important? They play a crucial role in regulating the activity of enzymes. Imagine you have a powerful tool, like a sharp knife. You wouldn’t want it to be constantly active, right? It could cause a lot of damage. Similarly, some enzymes are so potent that they could cause harm if they were always active. Zymogens provide a way to control their activity, ensuring they’re only activated when and where they’re needed.
Here’s a real-world example: Pepsinogen is a zymogen found in the stomach. It’s converted into pepsin, the active enzyme responsible for breaking down proteins in our food. Pepsinogen is only activated by the acidic environment in the stomach, making sure that protein digestion only occurs where it’s supposed to.
In short, zymogens are inactive enzyme precursors that provide a smart way for cells to control the activity of powerful enzymes, preventing unintended damage and ensuring that these critical processes happen at the right time and place.
Is pepsinogen a proenzyme?
Let’s break down what this means. Pepsinogen is the inactive form of the enzyme pepsin. Enzymes are proteins that speed up chemical reactions in our bodies. Pepsin is a digestive enzyme, specifically responsible for breaking down proteins in our stomachs.
Think of pepsinogen like a dormant superhero waiting to be activated. Pepsinogen is produced by specialized cells in the stomach lining called chief cells. When food enters the stomach, the stomach lining releases hydrochloric acid (HCl). This acidic environment lowers the pH, making the stomach contents very acidic. The acidic environment triggers the conversion of pepsinogen to pepsin. This change involves a small part of the pepsinogen molecule being cleaved off, revealing the active site of pepsin. Once activated, pepsin can then break down proteins into smaller peptides and amino acids, which are easier for our bodies to absorb.
This activation process ensures that pepsin is only active in the stomach where it’s needed. It prevents pepsin from prematurely digesting the stomach lining itself. The process of converting pepsinogen to pepsin is an example of how our bodies regulate enzyme activity and ensure optimal digestion.
What is another name for zymogen?
Think of it like this: a zymogen is a protein that’s ready to do something cool, but it needs a little nudge first. This nudge comes in the form of a chemical change called hydrolysis, where water is added. This change splits the protein into two parts, one of which is the active enzyme.
So, why do enzymes need this extra step? Well, it’s all about keeping things under control. If enzymes were constantly active, they could wreak havoc in your body. They need to be switched on only when and where they are needed.
Let’s break this down with a real-world example. Imagine you’re baking a cake. The flour, eggs, and sugar are the ingredients, like the inactive zymogen. You need to combine these ingredients and bake them to create the final product, the active enzyme. Just like baking, the body carefully controls the activation of enzymes to ensure they do their jobs at the right time.
So, next time you hear someone talking about proenzymes or zymogens, remember they’re just inactive enzymes waiting for their chance to shine!
Is trypsinogen a zymogen?
Here’s a simple explanation: Think of a zymogen like an inactive ingredient that needs to be “turned on” to do its job. Trypsinogen is this inactive form, and it’s produced in the pancreas. When food enters the small intestine, a signal is sent to the pancreas to release trypsinogen. In the small intestine, another enzyme called enterokinase activates trypsinogen, transforming it into its active form, trypsin. Trypsin is a powerful digestive enzyme that breaks down proteins into smaller pieces that our bodies can absorb.
Think of it like this: Imagine a chef who wants to cook a delicious meal. They need to use specific ingredients, but these ingredients are stored in a special container, locked and inactive. The chef has a key to unlock the container and activate the ingredients. In this analogy, the trypsinogen is the inactive ingredient, the enterokinase is the key, and the trypsin is the activated ingredient ready to work its magic in the digestive process.
Is proinsulin a zymogen?
Zymogens, also known as proenzymes, are inactive precursor forms of enzymes. They require a specific proteolytic cleavage event to become active. While proinsulin does undergo proteolytic cleavage to become insulin, the process is not about activating an inactive enzyme.
Proinsulin is synthesized in the beta cells of the pancreas as a single polypeptide chain. This chain contains the A and B chains of insulin, connected by a C-peptide. The C-peptide is removed during the conversion of proinsulin to insulin. Proinsulin does not have any enzymatic activity, and the conversion to insulin is not about activating an enzyme. Instead, the cleavage removes the C-peptide and allows the A and B chains of insulin to fold correctly, forming the active hormone.
The reason why proinsulin is not considered a zymogen is that it is not an inactive precursor of an enzyme. It is a precursor of a hormone. Hormones are chemical messengers that regulate various bodily functions. They don’t have enzymatic activity, and their activity is not dependent on a proteolytic cleavage event.
See more here: What Is The Difference Between Zymogen And Pepsinogen? | Difference Between Proenzyme And Zymogen
What is the difference between proenzyme and zymogen?
Think of it like this: Proenzyme is the general term for any inactive enzyme precursor. It’s like a dormant seed that needs the right conditions to sprout. Zymogen is a more specific term used for proenzymes that are activated by proteolysis, a process where a protein is broken down into smaller pieces.
So, all zymogens are proenzymes, but not all proenzymes are zymogens. Got it?
Let’s break down the activation process of zymogens a little further:
Zymogens often have an extra section of amino acids, called a propeptide. This propeptide acts as a “lock” that prevents the enzyme from being active.
* When the body needs the enzyme to do its job, a specific protease (an enzyme that breaks down proteins) comes along and chops off the propeptide.
* This removal is like releasing the lock, transforming the zymogen into an active enzyme.
Why go through all this trouble of having inactive forms? Well, imagine if all your digestive enzymes were active all the time. They’d be digesting your own tissues! By keeping them inactive until they’re needed, the body ensures that they only work where and when they’re supposed to.
This activation process is super important in many biological processes. For example, in your digestive system, zymogens like pepsinogen and trypsinogen are activated to break down food. Similarly, in your blood clotting cascade, zymogens like prothrombin and fibrinogen are crucial for forming blood clots to stop bleeding.
Which enzyme is considered a zymogen?
But why are they inactive in the first place? It’s all about control. Imagine a powerful enzyme floating around freely inside your body. It could cause damage if it wasn’t carefully controlled. Zymogens act as a safety mechanism. They prevent these enzymes from causing harm until they’re needed.
To illustrate this, let’s look at digestive enzymes like pepsin and trypsin. They’re responsible for breaking down proteins in your food. But if they were active in your stomach lining, they’d digest your own tissues! That’s why they’re secreted as zymogens. They’re only activated when they reach the small intestine, where they can safely work on food.
Here’s a breakdown of how zymogens work:
Inactive Zymogen: The enzyme is in its inactive form, like a puzzle piece that hasn’t been put together.
Activation: A specific part of the zymogen’s structure is removed, like taking a key piece out of the puzzle.
Active Enzyme: The enzyme becomes active and can now perform its job.
Some examples of zymogens include:
Pepsinogen: This zymogen is activated in the stomach to become pepsin, which helps digest proteins.
Trypsinogen: This zymogen is activated in the small intestine to become trypsin, another important protein-digesting enzyme.
Chymotrypsinogen: This zymogen is also activated in the small intestine to become chymotrypsin, which helps break down proteins.
In general, destructive catalytic enzymes like digestive enzymes are secreted as zymogens. They’re like powerful tools that need to be carefully handled. The zymogen form allows for safe storage and transport until they’re ready to do their job.
What is a zymogen in biochemistry?
A zymogen needs to undergo a specific biochemical change to transform into an active enzyme. These changes can be things like a hydrolysis reaction where a part of the molecule is removed, revealing the active site. The active site is where the enzyme binds to its target molecule (substrate) to carry out its specific job. In other cases, the zymogen might need to change its shape or configuration to expose the active site. This transformation process can be triggered by various factors, like the presence of a specific molecule, a change in pH, or even the action of another enzyme.
So, why are zymogens so important? Well, they offer a great way to control enzyme activity and prevent unwanted reactions. For instance, imagine if digestive enzymes were active in the cells where they’re produced. They could digest the cell itself! By keeping them in their inactive zymogen form, our bodies ensure these powerful enzymes are only activated in the digestive tract where they’re needed.
Let’s take a closer look at some examples. Pepsinogen is the zymogen form of the digestive enzyme pepsin, which breaks down proteins in the stomach. When pepsinogen enters the stomach’s acidic environment, it undergoes a conformational change and transforms into the active pepsin. Another example is trypsinogen, the inactive form of the digestive enzyme trypsin, which is activated by enteropeptidase in the small intestine.
In short, zymogens are important molecules in biochemistry because they offer a smart and controlled way to regulate enzyme activity, ensuring the right enzyme is active in the right place at the right time. It’s all about keeping things balanced and ensuring smooth cellular processes.
How does a zymogen become an active enzyme?
The active site is the key part of the enzyme where the magic happens – where the enzyme binds to its substrate and catalyzes the reaction.
This activation process usually takes place in the Golgi bodies, where a specific part of the precursor enzyme is cleaved off. This removal of a portion of the zymogen exposes the active site, like removing a piece of a puzzle that was blocking the whole picture. This allows the enzyme to fold into its correct three-dimensional shape, which is essential for its activity.
Let’s break down the example of hydrolysis a bit further. Hydrolysis is a chemical reaction where water is used to break down a molecule. In the case of zymogens, hydrolysis can break a specific bond in the enzyme, removing a portion of the molecule and revealing the active site. This is similar to how a chef uses water to soften tough cuts of meat, making them more tender and ready for cooking.
There are many different examples of zymogens in the body, such as pepsinogen (the inactive form of pepsin, an enzyme that breaks down proteins in the stomach), trypsinogen (the inactive form of trypsin, an enzyme that further breaks down proteins in the small intestine), and chymotrypsinogen (the inactive form of chymotrypsin, another enzyme that breaks down proteins in the small intestine).
Each of these zymogens undergoes a specific activation process, involving hydrolysis, to become their active forms, enabling them to perform their crucial roles in digestion. These enzymes work together, like a well-coordinated team, to break down the food we eat into smaller molecules that our bodies can absorb and use for energy and growth.
See more new information: bmxracingthailand.com
Difference Between Proenzyme And Zymogen: What’S The Difference?
Let’s start with the basics. Both proenzymes and zymogens are inactive forms of enzymes, meaning they can’t do their job of speeding up chemical reactions just yet. Think of them like a locked door – they’re there, but you need the right key to open them.
The key, in this case, is usually a specific chemical change. This change can happen in a few different ways:
Cleavage: The proenzyme or zymogen gets cut at a specific spot, removing a piece that was blocking its active site.
Modification: A molecule gets added or removed from the proenzyme or zymogen, changing its shape and making it active.
Binding: Something binds to the proenzyme or zymogen, causing a conformational change that unlocks its activity.
Now, you might be wondering, “What’s the difference between the two then?” Well, it’s a bit of a gray area, and the terms are often used interchangeably. But here’s the general idea:
Zymogen: This term usually refers to inactive forms of enzymes involved in digestive processes. Think of pepsinogen, which is the inactive form of pepsin, an enzyme that breaks down proteins in your stomach.
Proenzyme: This term is more general and can be used for inactive forms of any type of enzyme. So, pepsinogen is a zymogen, but it’s also a proenzyme.
The main takeaway is that both terms refer to inactive forms of enzymes, and they both become active through a specific chemical change.
To make things clearer, let’s look at some examples:
Zymogens in Digestion:
Pepsinogen: Inactive form of pepsin, a protein-digesting enzyme in the stomach. It’s activated by hydrochloric acid (HCl) in the stomach.
Trypsinogen: Inactive form of trypsin, a protein-digesting enzyme in the small intestine. It’s activated by enteropeptidase, another enzyme found in the small intestine.
Chymotrypsinogen: Inactive form of chymotrypsin, another protein-digesting enzyme in the small intestine. It’s activated by trypsin.
Proenzymes in Other Processes:
Prothrombin: Inactive form of thrombin, a clotting factor in blood. It’s activated by thromboplastin in a cascade of reactions.
Plasminogen: Inactive form of plasmin, an enzyme that breaks down blood clots. It’s activated by tissue plasminogen activator (tPA) and urokinase, among other factors.
As you can see, proenzymes and zymogens are essential for regulating important biological processes. They prevent enzymes from being active when they’re not needed, which helps to keep everything running smoothly.
Let’s tackle some frequently asked questions about proenzymes and zymogens:
FAQs
1. What is the difference between a proenzyme and an enzyme?
A proenzyme is an inactive form of an enzyme. An enzyme, on the other hand, is the active form of the protein that can catalyze a specific chemical reaction.
2. What are some examples of zymogens?
Some examples of zymogens include pepsinogen, trypsinogen, chymotrypsinogen, prothrombin, and plasminogen.
3. Why are proenzymes important?
Proenzymes are important because they help to regulate biological processes. They prevent enzymes from being active when they’re not needed, which helps to keep everything running smoothly. For example, if pepsin were active in the stomach all the time, it could digest the stomach lining itself!
4. How are proenzymes activated?
Proenzymes are activated by specific chemical changes. These changes can include cleavage, modification, or binding.
5. What is the difference between a proenzyme and a precursor?
The terms “proenzyme” and “precursor” are often used interchangeably. However, “precursor” is a more general term that refers to any molecule that is transformed into another molecule.
6. Can proenzymes be activated by heat?
In some cases, heat can activate proenzymes, but this is not the primary mechanism of activation. Heat can denature proteins, which might lead to a change in their activity.
7. Are proenzymes found in all organisms?
Yes, proenzymes are found in all living organisms. They play crucial roles in regulating many important biological processes.
8. What is the role of zymogens in digestion?
Zymogens play a critical role in digestion by breaking down large food molecules into smaller, more digestible units. This process is essential for extracting nutrients from food.
9. What happens if a proenzyme is not activated correctly?
If a proenzyme is not activated correctly, it may not be able to perform its function. This could lead to a range of problems, depending on the specific enzyme involved.
10. Are there any diseases associated with proenzymes?
Yes, there are several diseases associated with proenzymes, such as hemophilia and cystic fibrosis.
I hope this breakdown helped you understand the difference between proenzymes and zymogens. If you have any more questions, feel free to ask!
Zymogen | Enzymes, Activation, Proteins | Britannica
Zymogen, any of a group of proteins that display no catalytic activity but are transformed within an organism into enzymes, especially those that catalyze reactions involving the Britannica
Proenzyme vs Zymogen – What’s the difference? | WikiDiff
In biochemistry terms the difference between proenzyme and zymogen is that proenzyme is any inactive precursor of an enzyme that is converted to an enzyme by proteolysis; a WikiDiff
6.6: Enzymes and Protein Regulation – Biology LibreTexts
A zymogen also called a proenzyme, is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction Biology LibreTexts
Zymogen | Definition, Activation & Granules – Lesson
A zymogen also termed a proenzyme, is a dormant enzyme activated when a portion of the protein is cleaved, either chemically/or enzymatically. Zymogens Study.com
Structural Biochemistry/Enzyme/Zymogen – Wikibooks
A zymogen(also denoted as a proenzyme) is a group of proteins that can also be described as an inactive enzyme. Since it is an inactive precursor, it does not wikibooks.org
Molecular mechanisms for the conversion of zymogens to active …
Proteolytic enzymes are synthesized as inactive precursors, or “zymogens,” to prevent unwanted protein degradation, and to enable spatial and temporal regulation of National Center for Biotechnology Information
Zymogen – an overview | ScienceDirect Topics
Early activation of these zymogens, before their arrival to the intestinal tract, may cause damage to the organs they are released from. Other zymogens are present in plasma ScienceDirect
Zymogen – Knowledge and References – Taylor & Francis
Zymogen is a term used in biochemistry to refer to an inactive precursor of an enzyme. It is the state in which an enzyme may be synthesized and is typically activated by specific Taylor & Francis
Zymogen – Hemostasis.com
A zymogen (or proenzyme) is an inactive enzyme precursor. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or hemostasis.com
Zymogen Activation | What Is A Zymogen | Proteolytic Activation | Peptide Cleavage | Proenzymes |
What Is A Zymogen? Introduction To Pancreatic Enzymes Easy To Understand
Zymogens | Pro-Enzymes | Precursor Enzymes | Enzymology Lecture 05 | Educational Scholar
Zymogen Activation
Proenzymes Isozymes And Zymogens
What Are Proenzymes (Zymogens) ? | 11 | Biomolecules | Biology | Pradeep | Doubtnut
Basics Of Enzymology||Zymogens||
What Is The Role Of Zymogen? Why Is It An Inactive Precursor?
Link to this article: difference between proenzyme and zymogen.
See more articles in the same category here: https://bmxracingthailand.com/what
