Let’s discuss the question: how many unpaired electrons does zirconium have. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.
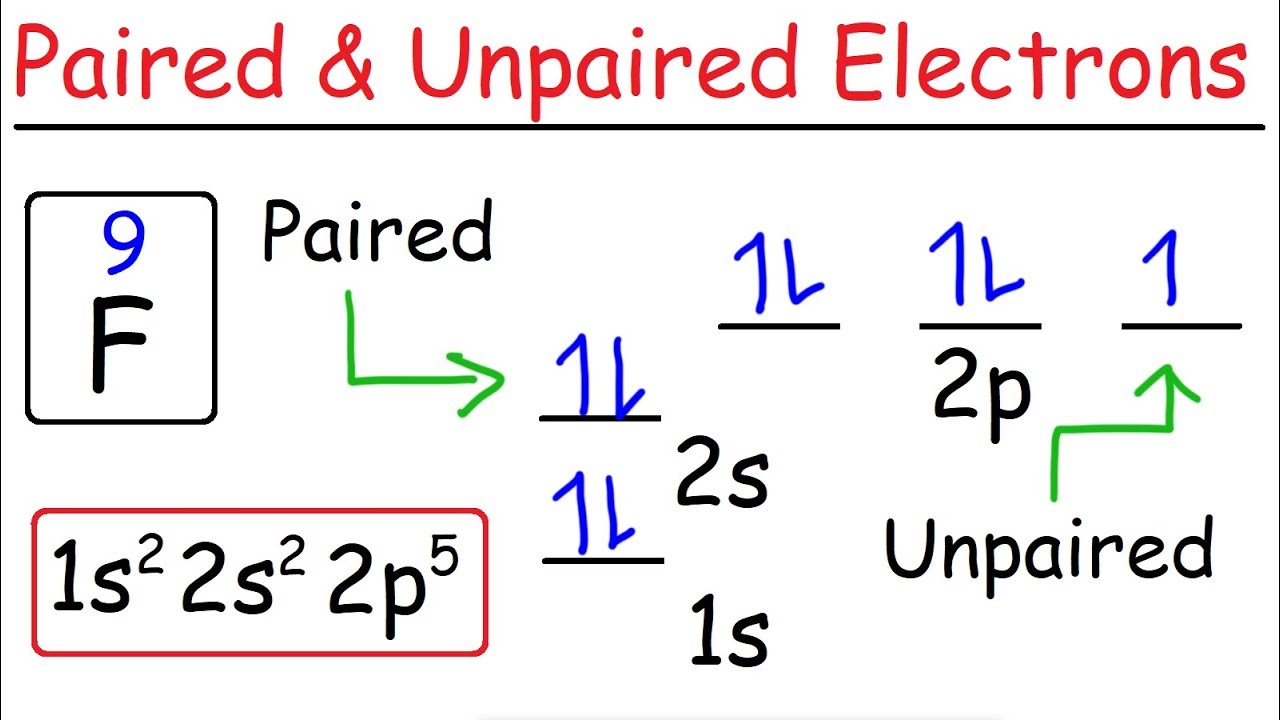
How many electrons do zirconium have?
Zirconium has 40 electrons and 40 protons, so this picture has 40 electrons separated into the different levels.
How do you find unpaired electrons?
Complete answer:
For finding the number of unpaired electrons, then first we have to find the atomic number of the element then write the configuration in the ground state, then according to the oxidation state subtract the number of electrons from the outer shell. So, there are 4 unpaired electrons.
How To Determine The Number of Paired and Unpaired Electrons
Images related to the topicHow To Determine The Number of Paired and Unpaired Electrons

How many valence electrons does zirconium have?
| Atomic Number | 40 |
|---|---|
| Electron Configuration | [Kr] 4d2 5s2 |
| Valence Electrons | 4d2 5s2 |
| Oxidation State | -2 1;2;3;4 |
| Atomic Term Symbol (Quantum Numbers) | 3F2 |
How many unpaired electrons does be have?
A beryllium atom will have no unpaired electrons.
We determine this by looking at the electron configuration of beryllium.
How many protons does zirconium have?
How are the electrons arranged in zirconium?
Zirconium atoms have 40 electrons and the shell structure is 2.8. 18.10. 2. The ground state electron configuration of ground state gaseous neutral zirconium is [Kr].
How many unpaired electrons does carbon have?
Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
Which element has 6 dots in its electron dot diagram?
Explanation: The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen.
What is paired and unpaired electrons?
Paired electrons are the electrons in an atom that occur in an orbital as pairs whereas unpaired electrons are the electrons in an atom that occur in an orbital alone. Therefore, paired electrons always occur as a couple of electrons while unpaired electrons occur as single electrons in the orbital.
Does zirconium have 4 valence electrons?
The electron configuration shows that the last shell of zirconium has two electrons and the d-orbital has a total of two electrons. Therefore, the valence electrons of zirconium(Zr) are four.
How many neutrons does zirconium have?
Typical zirconium contains about 50 neutrons, but ⁸⁸Zr, which is radioactive and not found naturally on Earth, has fewer than normal, with 48 neutrons.
How many orbitals does zirconium have?
Therefore, a zirconium atom will have two electrons in the first shell, eight in the 2nd orbit, eighteen electrons in the 3rd shell, ten electrons in the 4th shell, and the remaining two electrons will be in the 5th shell.
How to Write the Electron Configuration for Zirconium (Zr)
Images related to the topicHow to Write the Electron Configuration for Zirconium (Zr)
What is Group 5A called?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
How many unpaired electrons does B+ have?
…
How many paired and unpaired electrons are in boron?
| Element | Atomic number | Electron configuration |
|---|---|---|
| nitrogen | 7 | 1s22s22p3 |
What is unpaired valence electron?
Unpaired electrons are electrons in an orbital which are are alone, while valence electrons are just electrons that are in the outermost shell of an atom.
What is the protons neutrons and electrons of zirconium?
The nucleus consists of 40 protons (red) and 50 neutrons (blue). 40 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Zirconium is a transition metal in group 4, period 5, and the d-block of the periodic table. It has a melting point of 1855 degrees Celsius.
What group is zirconium in?
| Group | 4 | 1854°C, 3369°F, 2127 K |
|---|---|---|
| Period | 5 | 4406°C, 7963°F, 4679 K |
| Block | d | 6.52 |
| Atomic number | 40 | 91.224 |
| State at 20°C | Solid | 90Zr, 92Zr, 94Zr |
What is zirconium on the periodic table?
zirconium (Zr), chemical element, metal of Group 4 (IVb) of the periodic table, used as a structural material for nuclear reactors.
What is the atomic structure of zirconium?
The nucleus consists of 40 protons (red) and 51 neutrons (orange). 40 electrons (white) successively occupy available electron shells (rings). Zirconium is a transition metal in group 4, period 5, and the d-block of the periodic table. It has a melting point of 1855 degrees Celsius.
How many electrons are in an atom of zirconium with a charge of 3?
There are 40 protons, 51 neutrons, and 40 electrons in the zirconium atom. This is an atom which has no overall charge.
How many protons does krypton have?
Is carbon 4 unpaired electrons?
According to this theory, when the carbon atom is in an excited state, one of the two electrons located in the 2s orbital will get promoted to the empty 2pz orbital. As a result, carbon now has 4 unpaired valence electrons with which it can form four bonds.
Unpaired electrons
Images related to the topicUnpaired electrons

Does carbon have 4 valence electrons?
Atomic carbon has six electrons: two inner shell (core) electrons in the 1s orbital, and four valence (outer most shell) electrons in the 2s and 2p orbitals.
What is the electron arrangement for carbon?
Related searches
- how many unpaired electrons does s have
- zr electron configuration
- how many unpaired electrons does zr4+ have
- how many valence electrons are in zirconium
- how many 4d electrons are in zirconium
- how many unpaired electrons does ge have
- how many unpaired electrons does ‘s have
- how many unpaired electrons does zr4 have
- how many unpaired electrons does si have
- how many electrons are in zirconium
- how many unpaired electrons does o have
- how many unpaired electrons does p have
- how many unpaired electrons in silver
- how many unpaired electrons does cu2+ have
- how many unpaired electrons does mg have
- how many unpaired electrons in zr
- how many negative electrons does zirconium have
Information related to the topic how many unpaired electrons does zirconium have
Here are the search results of the thread how many unpaired electrons does zirconium have from Bing. You can read more if you want.
You have just come across an article on the topic how many unpaired electrons does zirconium have. If you found this article useful, please share it. Thank you very much.
