What will happen if KCl is added to water?
It’s important to understand that potassium chloride doesn’t chemically react with water to form new substances. It simply dissolves and separates into its ions. Think of it like sugar dissolving in water – the sugar molecules disperse throughout the water but don’t change their chemical composition.
Here’s a closer look at what happens:
Dissolving:Potassium chloride is an ionic compound, meaning it’s made of positively charged potassium ions and negatively charged chloride ions held together by electrostatic forces. When you add potassium chloride to water, the water molecules, which are polar, attract these ions. The positive end of a water molecule (the hydrogen atom) is attracted to the negative chloride ions, and the negative end (the oxygen atom) is attracted to the positive potassium ions.
Ionization: These attractions are strong enough to overcome the forces holding the potassium and chloride ions together in the potassium chloride crystal. The ions separate and become surrounded by water molecules, forming what we call hydrated ions.
This process is why potassium chloride solutions can conduct electricity. The free-moving ions can carry an electric current.
Why does KCl dissolve in water but not CH4?
KCl and NaCl are ionic compounds, meaning they’re formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Water is a polar solvent due to the uneven sharing of electrons between the hydrogen and oxygen atoms. This creates a partial positive charge on the hydrogen side and a partial negative charge on the oxygen side.
When KCl is added to water, the positively charged K+ ions are attracted to the negative side of the water molecules, and the negatively charged Cl- ions are attracted to the positive side. These attractions overcome the forces holding the KCl together, causing it to dissolve. This process is called hydration.
CH4, on the other hand, is a nonpolar covalent compound. The electrons are shared equally between the carbon and hydrogen atoms, making the molecule have no overall charge. Water is unable to form strong enough attractions with the CH4 molecules to overcome the forces holding the CH4 together. This is why CH4 doesn’t dissolve in water.
Think of it this way: water is like a bunch of magnets, attracting and holding onto other magnets. Ionic compounds are like magnets because they have a positive and negative side. But nonpolar compounds are like non-magnetic objects; they can’t interact with magnets (the water molecules).
Solubility is all about how strong the attractive forces between the solute and solvent are compared to the forces holding the solute together. In the case of KCl, the attraction between the ions and water molecules is stronger than the ionic bonds holding KCl together, leading to dissolution. In the case of CH4, the attraction between CH4 and water molecules is weak, so it stays undissolved.
Why is KCl more soluble in hot water?
Temperature plays a key role in how much of a solid can dissolve in a liquid. Think of it like this: when you heat up water, you’re essentially giving its molecules more energy. These energized water molecules become more “active” and can wiggle their way between the KCl molecules, pulling them apart and into the solution.
KCl is considered a soluble salt, which means it likes to hang out in water. But just like any friendship, there are factors that influence how close they get. In this case, temperature is one of those key factors. As the water gets hotter, the KCl molecules have more energy to break free from the solid and join the water party, leading to a greater amount of KCl dissolving.
Here’s a more detailed explanation:
Increased Molecular Motion: When you heat water, the water molecules start moving faster and with more energy. This increased motion makes it easier for them to break apart the KCl crystal lattice.
Weakening of Intermolecular Forces: KCl crystals are held together by strong ionic bonds. These bonds are attractive forces between the positively charged potassium ions (K+) and the negatively charged chloride ions (Cl-). As the temperature increases, the water molecules bump into the KCl crystal lattice with more energy, weakening these ionic bonds. This weakening makes it easier for the water molecules to pull the ions away from the crystal.
Enthalpy of Dissolution: The process of dissolving KCl in water is endothermic. This means that energy is absorbed during the process. When you add heat to the system, you are providing the energy needed to overcome the endothermic nature of the dissolving process, further encouraging the KCl to dissolve.
In essence, increasing the temperature gives the water molecules more “oomph” to break apart the KCl and form a solution. It’s like giving your friends a little extra energy so they can break free from a group and join you for a fun time!
Is H2O a good solvent for KCl?
Let’s break down why KCl dissolves well in water. KCl is an ionic compound, meaning it’s made up of positively charged potassium ions (K+) and negatively charged chloride ions (Cl-). Water molecules are also polar, with a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). When KCl is added to water, the positive ends of the water molecules attract the negative chloride ions, while the negative ends of the water molecules attract the positive potassium ions. This attraction overcomes the forces holding the KCl crystal together, causing the KCl to dissolve.
CCl4, on the other hand, is a nonpolar molecule. The carbon and chlorine atoms have similar electronegativity values, so the electrons are shared almost equally. This means there’s no significant charge separation within the molecule. Since CCl4 is nonpolar, it cannot effectively interact with the polar KCl ions. As a result, KCl does not dissolve well in CCl4.
In summary, KCl is more soluble in water because both are polar and can interact favorably through electrostatic interactions. CCl4, being nonpolar, does not have the same attraction to the polar KCl ions, and therefore does not dissolve it well.
Does water dissolve potassium?
For example, potassium dichromate can dissolve up to 115 grams per liter of water, potassium permanganate up to 76 grams per liter, potassium iodide up to 92 grams per liter, and potassium chloride up to 1480 grams per liter.
So, while potassium as a pure element won’t dissolve, many of its compounds are quite soluble in water.
Here’s a deeper dive into the science behind this:
When we talk about dissolving, we’re really talking about a chemical reaction. The water molecules surround the potassium compound molecules and break them apart, forming ions in the solution. This happens because the water molecules are polar, meaning they have a slightly positive end and a slightly negative end. This polarity allows them to interact with the charged ions of the potassium compound, pulling them apart and keeping them dissolved.
The solubility of a potassium compound in water is influenced by a few factors, including:
The type of anion (the negatively charged ion) in the compound. Some anions, like chloride, are more attracted to water molecules than others, making the compound more soluble.
Temperature. Generally, higher temperatures increase the solubility of most potassium compounds in water. This is because the increased energy helps break apart the compound molecules and facilitates dissolving.
It’s important to remember that not all potassium compounds are equally soluble. Some, like potassium permanganate, are readily soluble, while others, like potassium sulfate, have more limited solubility in water.
How to dissolve potassium chloride?
Why does potassium chloride dissolve in water?
Potassium chloride (KCl) is an ionic compound, meaning it’s made up of positively charged potassium ions (K+) and negatively charged chloride ions (Cl-). When you add KCl to water, the water molecules surround the ions and pull them apart. This process is called hydration, and it’s driven by the attraction between the positive and negative charges of the ions and water molecules.
Why is it important to stir the solution?
Stirring the solution helps to speed up the dissolution process. The stirring action helps to distribute the potassium chloride throughout the water and brings fresh water molecules into contact with the undissolved tablet. This increases the rate at which the ions are hydrated and pulled away from the tablet.
What happens to the potassium chloride after it dissolves?
Once the potassium chloride dissolves, the ions become evenly distributed throughout the solution. These ions are then available to be absorbed by the body, where they play important roles in maintaining electrolyte balance, muscle function, and nerve transmission.
Can I dissolve potassium chloride in other liquids besides water?
While water is the most common solvent for potassium chloride, it can also be dissolved in other liquids, such as alcohol. However, it’s important to note that the solubility of potassium chloride in different liquids varies. For example, KCl is much more soluble in water than it is in alcohol.
What about dissolving potassium chloride in food?
It’s not recommended to dissolve potassium chloride in food. This is because the saltiness of the potassium chloride might make the food taste unpleasant, and it could also lead to an uneven distribution of the potassium chloride in the food. If you are taking potassium chloride supplements, it’s best to take them according to the instructions on the label, which typically involve dissolving them in water.
Does KCl hydrolyze in water?
KCl, or potassium chloride, is formed when the strong base potassium hydroxide (KOH) reacts with the strong acid hydrochloric acid (HCl). Because both the cation (K+) and the anion (Cl-) are derived from strong electrolytes, they don’t react with water. This means the solution remains neutral, and no hydrolysis occurs.
Let’s dive a little deeper into hydrolysis. It’s basically the reaction of a salt with water. The salt breaks down into its ions, and then one or both of these ions can react with water molecules. This reaction can make the solution acidic, basic, or leave it neutral, depending on the nature of the ions.
Now, here’s the key: hydrolysis is more likely to occur when either the cation or anion is derived from a weak acid or a weak base. For example, if we look at sodium acetate (CH3COONa), the acetate ion (CH3COO-) is the conjugate base of a weak acid (acetic acid). When sodium acetate dissolves in water, the acetate ion reacts with water, forming acetic acid (CH3COOH) and hydroxide ions (OH-). This makes the solution slightly basic.
But remember, KCl is different. It’s formed from a strong base and a strong acid, so neither the potassium ion nor the chloride ion reacts with water. This means the solution remains neutral, and no hydrolysis takes place.
See more here: What Will Happen If Kcl Is Added To Water? | Does Kcl Dissolve In Water
Is KCl soluble in water?
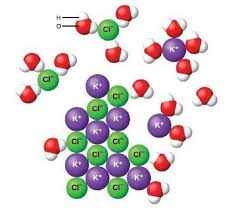
When potassium chloride dissolves, it breaks apart into its individual ions: potassium ions (K+) and chloride ions (Cl-). This process is called dissociation. Potassium chloride’s solubility in water is due to the strong attraction between the positively charged potassium ions and the negatively charged oxygen atoms in water molecules. Similarly, the negatively charged chloride ions are attracted to the positively charged hydrogen atoms in water molecules. These attractions overcome the forces holding the potassium chloride crystal together, allowing it to dissolve.
Think of it this way: Water molecules act like little magnets, pulling the potassium chloride apart. This process of dissociation is a key property of soluble ionic compounds like potassium chloride. It’s important to remember that the dissociation of potassium chloride is not just a simple physical change. It’s a chemical process where the ionic bonds within the potassium chloride crystal are broken, and new interactions form between the ions and water molecules.
Here’s a little more detail about why potassium chloride dissolves so well:
Polarity: Water is a polar molecule, meaning it has a slightly positive end and a slightly negative end. This polarity allows water molecules to interact strongly with the charged ions of potassium chloride.
Ionic Bonds: Potassium chloride is an ionic compound formed by the electrostatic attraction between potassium ions (K+) and chloride ions (Cl-). These ionic bonds are strong, but they can be overcome by the strong attractions between the ions and water molecules.
Let me know if you’d like to learn more about potassium chloride’s properties or how its solubility impacts its uses!
What happens when KCl is dissolved in water?
When KCl is added to water, it dissociates into its ions, potassium (K+) and chloride (Cl-). These ions are surrounded by water molecules, which is why we indicate this with (aq), meaning aqueous (dissolved in water).
Here’s the chemical equation that represents this process:
KCl(s) + H2O(l) → K+(aq) + Cl-(aq)
The (s) indicates that KCl is a solid, and the (l) indicates that water is a liquid.
Let’s break down why this happens:
Water is a polar molecule – one end of the molecule has a slightly positive charge, while the other end has a slightly negative charge. This polarity allows water molecules to interact with KCl’s ions. The positively charged hydrogen end of water molecules will be attracted to the negatively charged chloride ions, and the negatively charged oxygen end of water molecules will be attracted to the positively charged potassium ions.
This attraction between the water molecules and the KCl ions is strong enough to overcome the attractive forces holding the KCl ions together in the solid crystal. As a result, the KCl dissolves, and the K+ and Cl- ions become surrounded by water molecules, effectively becoming hydrated.
This process of dissolving is also known as solvation, and it’s a common phenomenon for many ionic compounds when they are mixed with water.
What is the solubility of potassium chloride (KCl)?
Here’s a table showing the solubility of KCl in grams per 100 milliliters (g/100 mL) at different temperatures in degrees Celsius (°C):
| Temperature (°C) | Solubility (g/100 mL) |
|—|—|
| 0 | 27.6 |
| 10 | 31.0 |
| 20 | 34.0 |
| 30 | 37.0 |
| 40 | 40.0 |
| 50 | 43.0 |
| 60 | 46.0 |
| 70 | 49.0 |
| 80 | 52.0 |
| 90 | 55.0 |
| 100 | 58.0 |
What does this mean?
Essentially, the higher the temperature, the more KCl you can dissolve in a given amount of water. This is a common characteristic of many salts.
Think of it like a sponge. A cold sponge can only soak up a certain amount of water. But if you warm it up, it can absorb more. The same principle applies to water and KCl.
Why is solubility important?
Solubility is important for many reasons. For example, it determines how easily KCl can be used in various applications. These applications include:
Fertilizers: KCl is a key component of many fertilizers, providing potassium, a crucial nutrient for plant growth.
Medicine: KCl is used in intravenous solutions to replenish electrolytes in patients who are dehydrated or have lost potassium.
Food: KCl is used as a salt substitute in some food products.
Industrial processes: KCl is used in the production of various chemicals, plastics, and other materials.
Understanding the solubility of KCl helps us determine the right conditions for using it in these applications.
Is potassium chloride soluble in water?
When potassium chloride dissolves, it breaks down into its individual ions: potassium ions (K+) and chloride ions (Cl-). These ions become surrounded by water molecules, forming a solution. This process, called dissociation, happens because the attraction between the water molecules and the ions is stronger than the attraction between the potassium and chloride ions in the solid potassium chloride.
Think of it like this: potassium chloride is like a group of friends tightly holding hands. Water molecules, on the other hand, are friendly and love to make new connections. When water molecules meet the potassium chloride friends, they pull them apart, each water molecule grabbing an individual potassium or chloride ion. These individual ions then become surrounded by water molecules, floating freely in the solution.
The solubility of potassium chloride is actually quite high. This means that a lot of potassium chloride can dissolve in water before the solution becomes saturated, meaning that no more potassium chloride can dissolve.
This solubility is influenced by several factors, including temperature. As the temperature increases, the solubility of potassium chloride in water also increases. This means that more potassium chloride can dissolve in warmer water.
Understanding the solubility of potassium chloride is important in a variety of fields, from chemistry and biology to agriculture and medicine.
For instance, in agriculture, potassium chloride is used as a fertilizer to provide potassium, an essential nutrient for plant growth. In medicine, potassium chloride is used to treat low potassium levels in the blood.
So, the next time you see potassium chloride mentioned, remember that it readily dissolves in water, playing a significant role in many different areas!
See more new information: bmxracingthailand.com
Does Kcl Dissolve In Water: A Simple Explanation
Let’s break down why that happens.
KCl and Water: A Match Made in Chemistry
KCl, also known as potassium chloride, is a ionic compound. This means it’s made up of positively charged potassium ions (K+) and negatively charged chloride ions (Cl-). Water, on the other hand, is a polar molecule, meaning it has a slightly positive end and a slightly negative end.
Now, here’s where the magic happens. The positive end of a water molecule is attracted to the negative chloride ions in KCl, while the negative end of a water molecule is attracted to the positive potassium ions. These attractions are strong enough to overcome the forces holding the KCl crystals together, causing them to break apart and dissolve in the water.
Solubility: The Key to Understanding How Much Dissolves
The amount of KCl that dissolves in water at a given temperature is called its solubility. Solubility is a measure of how much of a substance can dissolve in a specific amount of solvent (in this case, water).
KCl is considered highly soluble in water, meaning it can dissolve a large amount of it. This solubility changes with temperature, though. The warmer the water, the more KCl it can hold.
Why KCl Dissolves: A Deeper Dive
Polarity and Attraction: The polar nature of water plays a crucial role in dissolving ionic compounds like KCl. The positive and negative ends of the water molecules effectively surround and pull apart the KCl ions, disrupting the crystal structure and leading to dissolution.
Hydration: Once the KCl ions are separated, they become surrounded by water molecules. This process is called hydration, and it further stabilizes the dissolved ions.
Entropy: You can also think about the process from a thermodynamic perspective. Dissolving KCl in water actually increases the entropy of the system, meaning there’s more disorder. This is a favorable outcome from a thermodynamic perspective, contributing to the dissolution process.
KCl in Action
So, why is this all important? Well, KCl has a variety of applications, and its solubility in water is key to many of them.
Fertilizers: KCl is a major component in fertilizers because plants need potassium for healthy growth. The high solubility of KCl means it can easily be absorbed by plant roots.
Salt Substitute: You might find KCl labeled as “salt substitute” in the grocery store. It has a similar salty taste to NaCl (table salt) but contains potassium instead of sodium. This can be helpful for people who need to limit their sodium intake for health reasons.
Medical Uses: KCl is also used in medicine. It can be given intravenously to treat low potassium levels in the blood, a condition called hypokalemia.
Industrial Uses: KCl has various industrial uses, including the production of potassium hydroxide, chlorine, and potassium permanganate.
KCl and its Impact on Your Health
You might be wondering about the health implications of KCl. While it’s an essential nutrient, too much potassium can be dangerous. However, the KCl you encounter in food or salt substitutes is unlikely to cause problems unless you have specific health conditions.
If you have any concerns about potassium intake or the use of KCl for health reasons, talk to your doctor.
FAQs
Here are some common questions about KCl and its solubility in water:
1. What happens to the dissolved KCl?
When KCl dissolves in water, the potassium ions (K+) and chloride ions (Cl-) are surrounded by water molecules. These hydrated ions remain dissolved in the solution unless the water evaporates, in which case the KCl would precipitate out.
2. Can KCl be undissolved?
Yes, you can get KCl out of solution. Heating the water will cause some of it to evaporate, increasing the concentration of KCl in the remaining solution. If you continue heating the solution, eventually all the water will evaporate, leaving behind solid KCl crystals.
3. How does temperature affect KCl’s solubility?
The solubility of KCl in water increases with temperature. This means that warmer water can dissolve more KCl than colder water.
4. Is it safe to drink water with dissolved KCl?
The amount of KCl in drinking water is typically very low. However, it’s always best to consult with your doctor if you have concerns about the potential health effects of KCl in your drinking water.
5. What’s the chemical equation for KCl dissolving in water?
The chemical equation for KCl dissolving in water is:
“`
KCl(s) -> K+(aq) + Cl-(aq)
“`
s = solid, aq = aqueous (dissolved in water)
This equation shows that when KCl dissolves in water, it breaks apart into potassium ions and chloride ions, which are surrounded by water molecules.
I hope this article has given you a deeper understanding of why KCl dissolves in water. If you have any other questions, feel free to ask!
Is KCl Soluble or Insoluble in Water? – YouTube
Is KCl (Potassium chloride) soluble or insoluble in water? The answer is that it is soluble in water. It is an ionic compound which readily dissociates into … YouTube
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O)
In this video we will describe the equation KCl + H2O and write what happens when KCl is dissolved in water. When KCl is dissolved in H2O (water) it will dissociate YouTube
4.10: Aqueous Solutions and Solubility – Compounds
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and Chemistry LibreTexts
Comprehensive Guide to Potassium Chloride Solubility
Potassium chloride (KCl) is a water-soluble salt with a grade of approximately 0-0.61. Its solubility in water increases with temperature, as shown in Experiment 4a of techiescience.com
The Comprehensive Guide to KCl Solubility: Mastering the
The Solubility of KCl in Water. The solubility of KCl in water at room temperature (25°C) is approximately 342 g/L (grams per liter). This value can vary techiescience.com
11.2: Electrolytes – Chemistry LibreTexts
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and Chemistry LibreTexts
9.1: Aqueous Solutions and Solubility: Compounds
These attractions play an important role in the dissolution of ionic compounds in water. Figure 9.1.2: As potassium chloride (KCl) dissolves in water, the Chemistry LibreTexts
Q: Is potassium chloride (KCl) soluble in water? – CK-12 Foundation
Yes, potassium chloride (KCl) is soluble in water. It readily dissolves, making a solution of potassium ions (K+) and chloride ions (Cl-). CK-12 Foundation
Potassium chloride | Definition, Formula, Uses, Flame
The water dissolves the potassium chloride, and this solution is then pumped back to the surface, where the water evaporates, leaving the potassium chloride. Uses. Potassium chloride is most often Britannica
Is Kcl Soluble Or Insoluble In Water?
Equation For Potassium Chloride Dissolving In Water ( Kcl + H2O)
Experiment 5: Potassium Chloride And Water
❤️Solvation Or Hydration || How Kcl ( Potassium Chloride ) Dissolved In Water👈 💧
How Water Dissolves Salt
How Solubility And Dissolving Work
Reaction Of Potassium And Water
When Potassium Chloride Is Dissolved In Water
Link to this article: does kcl dissolve in water.
See more articles in the same category here: https://bmxracingthailand.com/what
