Are hcp and CCP the same?
The main difference between HCP and CCP lies in the arrangement of their atomic layers. HCP has two layers of spheres in its repeating structure, while CCP has three. Think of it like stacking coins – in HCP, the second layer sits directly on top of the first, while in CCP, the second layer is shifted slightly to the side.
Let’s look at this a little closer:
HCP: Imagine you’re stacking a layer of spheres (think of them as coins). In the second layer, each sphere sits directly on top of a sphere in the first layer. This creates a repeating pattern where the spheres in each layer are directly above each other.
CCP: Here, the second layer’s spheres are positioned so that they sit in the “hollows” between the first layer’s spheres. The third layer then sits on top of the second, and its spheres are again positioned in the “hollows” between the spheres in the second layer.
The difference in stacking creates a slightly different overall structure. HCP has a hexagonal symmetry, whereas CCP has a cubic symmetry. However, both structures are very dense and stable, leading to them being common in a wide range of materials.
Some examples of materials with HCP structures include magnesium, zinc, and titanium. Materials with CCP structures include copper, silver, and gold.
While they might seem similar at first glance, understanding the subtle differences between HCP and CCP structures is important for understanding the properties and behavior of different materials.
What are the properties common to both hcp and CCP?
Let’s delve deeper into the similarities and differences between these two structures.
The hcp and ccp structures differ in the arrangement of their atomic layers. In the hcp structure, the layers are stacked in an ABABAB… pattern. Imagine stacking coins on top of each other. The first layer is labeled A, and the second layer is labeled B because it’s offset from the first layer. The third layer is then directly above the first layer, making it also an A layer. This pattern continues throughout the crystal.
In the ccp structure, the layers are stacked in an ABCABC… pattern. The third layer in the ccp structure is offset from both the first and second layer, making it a C layer. This pattern then continues throughout the crystal.
Despite this difference in stacking, both structures achieve the same coordination number and packing efficiency. This is because the arrangement of atoms in both structures maximizes the number of nearest neighbors and minimizes the amount of empty space.
Many common metals, like magnesium and zinc, adopt the hcp structure. Copper, silver, and gold are examples of metals that crystallize in the ccp structure. Their stability and high packing efficiency make these structures very common in nature.
What is the density of hcp structure?
Let’s break down what hcp means and how it relates to density. Imagine a bunch of spheres, like marbles, trying to fit together as tightly as possible. In an hcp structure, these spheres form layers where each sphere is surrounded by six other spheres in a hexagonal pattern. The second layer sits on the depressions formed by the first layer, with each sphere resting on three spheres in the first layer. The third layer is identical to the first layer, directly above it. This pattern continues, creating a tightly packed, three-dimensional structure.
The high packing density of hcp means that the atoms in the structure are very closely spaced, resulting in a high density. This is because there’s very little empty space between the atoms. So, when you hear about a material having an hcp structure, you can expect it to be quite dense. This is why many metals with hcp structures, like titanium, are strong and lightweight, making them desirable for applications like aerospace engineering.
Are fcc and CCP the same?
Imagine stacking spheres together as tightly as possible. There are different ways to do this, and one of them is called cubic close packing. In this arrangement, the spheres form a cube, with an atom at each corner and in the center of each face. This structure is also called face-centered cubic because of the atoms at the face centers.
So, when you see CCP or FCC, you’re talking about the same crystal structure. It’s like calling a person by their first name or their last name – both are correct!
The ABCABC Pattern
The ABCABC pattern you mentioned refers to the layers of atoms in a CCP structure. Let’s break it down:
Layer A: The first layer of atoms is arranged in a hexagonal pattern.
Layer B: The second layer sits on top of the first, with each atom fitting into the space between three atoms in the first layer. This creates a slightly offset arrangement.
Layer C: The third layer sits on top of the second layer, and its atoms are positioned directly above the atoms in the first layer.
Repeating Pattern: This ABCABC pattern continues, creating a tightly packed, three-dimensional structure.
Why is this important?
Understanding CCP and FCC is essential for studying materials science and chemistry. The crystal structure of a material affects its properties, such as its melting point, density, and conductivity.
Here’s an analogy: Think of building a tower with blocks. You could stack the blocks in different ways, and each way would create a different kind of tower with different strengths and weaknesses. The same is true for atoms in a crystal structure. The way they are arranged determines the properties of the material.
Do hcp and CCP have the same density?
While both hcp and ccp structures can be made up of the same element, they don’t necessarily have the same density. This is because they have a different number of atoms per unit cell. Hcp structures have 8 spheres per unit cell, whereas ccp structures have 4 spheres per unit cell.
Think of it like this: Imagine you have a box (the unit cell) and you’re packing marbles (the atoms) inside. In the hcp structure, you can fit more marbles into the box than in the ccp structure, even though the marbles themselves are the same size.
This difference in the number of atoms packed into the unit cell directly impacts the overall density. Density is calculated by dividing the mass by the volume. Since the hcp structure has more atoms in the same volume, its density will be higher compared to the ccp structure.
To illustrate this further, let’s consider a specific example:
Copper (Cu): Both hcp and ccp copper have the same atomic radius, but their density differs due to the packing efficiency. The hcp structure of copper has a density of 8.96 g/cm³, while the ccp structure has a density of 8.93 g/cm³. This difference in density, although seemingly small, arises directly from the varying number of atoms packed into their respective unit cells.
In essence, the density of a material depends not only on the atomic weight of the element but also on how efficiently its atoms are packed within the crystal structure. While both hcp and ccp structures are highly efficient in packing atoms, the difference in their packing arrangements leads to variations in density, even when composed of the same element.
Why CCP structure is more ductile than hcp structure?
Let’s break this down. Slip systems are essentially planes within the crystal structure where atoms can easily slide past each other under stress. Think of it like a deck of cards – you can slide the cards past each other more easily if they’re aligned in a specific way. The more slip systems a crystal structure has, the more ways it can deform without breaking.
FCC structures have a lot of slip systems because their atoms are packed in a very symmetrical way. This means there are many different directions the atoms can move under stress. BCC structures have fewer slip systems than FCC because their atoms are packed in a less symmetrical way. HCP structures have even fewer slip systems than BCC because they have a very specific arrangement of atoms that limits the directions they can move.
Imagine a material with only a few slip systems. If stress is applied, the atoms can only move in a limited number of ways, making it more likely to fracture. On the other hand, a material with many slip systems can deform more easily by allowing atoms to move in different directions. This is why FCC structures, with their high number of slip systems, are often more ductile than HCP structures.
What is the similarity between hcp and FCC?
First and foremost, both hcp and fcc structures are made up of identical atoms arranged in the same pattern within each layer. The difference lies in how these layers stack on top of each other. This stacking arrangement leads to the unique characteristics of each structure.
Another important similarity is their packing fraction, which is a measure of how efficiently the atoms are packed in the structure. Both hcp and fcc have the same packing fraction, which is the highest possible packing fraction for spheres, making them highly efficient structures.
The coordination number, which refers to the number of nearest neighbors surrounding an atom, is also the same for both hcp and fcc. In both structures, each atom is surrounded by 12 nearest neighbors, maximizing their interactions.
Now, let’s delve deeper into the differences between hcp and fcc. While both structures have the same atomic arrangement within each layer, the stacking sequence of these layers is different. This stacking sequence determines the overall symmetry and properties of the crystal. In hcp, the stacking sequence is ABABAB, meaning that the second layer (B) is directly above the first layer (A) and the third layer is directly above the second layer (B). In fcc, the stacking sequence is ABCABC, meaning that the third layer (C) is shifted relative to the first two layers (A and B). This seemingly small difference in stacking has a significant impact on the overall symmetry of the structure, leading to different properties for hcp and fcc.
For instance, hcp structures typically exhibit anisotropic properties, meaning their properties vary depending on the direction. This is because of the layered structure of hcp materials. In contrast, fcc structures are usually more isotropic, having similar properties in all directions. This is because the stacking sequence in fcc is more symmetrical than in hcp.
Which is more closely packed FCC or hcp?
Think of it like a honeycomb – those little hexagonal cells are the perfect way to pack spheres together tightly. This hexagonal arrangement is the most efficient way to pack spheres in two dimensions.
Why is this?
Well, imagine you’re trying to fit marbles into a box. If you just drop them in randomly, they’ll leave gaps between them. But if you arrange them in a hexagonal pattern, they fit snugly together. No wasted space!
Now, let’s consider the third dimension.
The hexagonal structure is the starting point for understanding how spheres pack in 3D. You can think of it as a layer of marbles, with each marble touching six others. But to create a three-dimensional structure, we need to add more layers.
This is where it gets interesting. There are two main ways to add these extra layers: face-centered cubic (FCC) and hexagonal close-packed (HCP).
In FCC, the second layer of spheres sits directly on top of the first layer, with each sphere fitting into a space between three spheres in the first layer. The third layer then sits directly on top of the second layer, creating a repeating pattern.
HCP, however, is a bit more complex. The second layer sits in the same way as FCC, with spheres filling the spaces between the first layer. But the third layer doesn’t sit directly above the first layer. It shifts slightly, so that each sphere in the third layer sits over a space between three spheres in the first layer. This creates a staggered arrangement.
Both FCC and HCP are very efficient ways of packing spheres in three dimensions. They both achieve the highest possible density of spheres, meaning they leave the least amount of empty space.
But how do we know which one is *more* closely packed?
That’s a tricky question, and it depends on how you define “closely packed.” Both FCC and HCP have the same packing density, meaning they take up the same amount of space when you fill a container with spheres. So, in that sense, they are equally closely packed.
However, there are some subtle differences in the arrangement of the spheres that could lead to different packing efficiencies depending on the specific application. But for our purposes, we can say that both FCC and HCP are incredibly efficient ways to pack spheres in three dimensions.
What is the main difference in the packing of spheres in hcp and CCP arrangement?
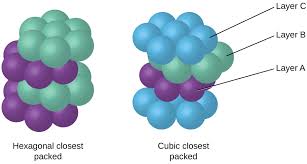
In hcp, the third layer aligns perfectly with the first layer, creating an AB-AB-AB pattern. This means that the spheres in the third layer are directly above the spheres in the first layer.
In ccp, the third layer is shifted, creating a ABC-ABC-ABC pattern. The spheres in the third layer are not directly above the spheres in the first layer. Instead, they are positioned above the spaces between the spheres in the first layer.
This difference in the third layer arrangement leads to distinct packing geometries and properties. Let’s explore these differences further.
Imagine stacking coins.
hcp is like stacking coins in a straight column, where each coin directly aligns with the one below it.
ccp is like stacking coins where each coin is shifted slightly, forming a staggered pattern. Think of a pyramid.
This difference in how the spheres are stacked gives hcp a hexagonal structure, while ccp gives a cubic structure.
Let’s talk about these structures in more detail.
hcp is characterized by its six-sided symmetry. This hexagonal symmetry is created by the arrangement of spheres in the first two layers. Think of a honeycomb.
ccp is characterized by its cubic symmetry. This cubic symmetry arises from the way the spheres are packed in the three layers. Think of a cube.
To understand the ABC-ABC-ABC pattern in ccp, consider a face-centered cubic (fcc) unit cell. Each corner of the cube has 1/8 of a sphere, and each face of the cube has 1/2 of a sphere. These spheres pack together in a way that creates the ABC-ABC-ABC stacking sequence.
The ccp arrangement maximizes the packing efficiency of spheres, meaning the spheres are packed as tightly as possible. This high packing efficiency leads to materials that are dense and strong.
For example, ccp is the most common packing arrangement for metals, such as copper, gold, and aluminum. This arrangement is also found in many other materials, such as carbon nanotubes and fullerenes.
hcp is also a highly efficient packing arrangement. However, it is not as efficient as ccp. This means that materials with hcp structures are typically slightly less dense than materials with ccp structures.
Metals like magnesium, zinc, and titanium often exhibit hcp arrangements.
In conclusion, the key difference between hcp and ccp lies in the stacking sequence of the third layer. hcp has a AB-AB-AB stacking sequence, while ccp has a ABC-ABC-ABC sequence. This difference leads to distinct packing geometries and properties, impacting the structure and properties of materials.
What is planar density in hcp?
The HCP (hexagonal close-packed) structure is a common crystal structure found in many metals like titanium. In the HCP structure, the (0001) plane is a particularly interesting one. This plane is the “base” of the hexagonal unit cell.
The planar density of the (0001) plane in HCP structures is calculated using the following formula:
PD = 1 / (3 * R^2)
Where:
PD is the planar density
R is the atomic radius
Let’s break down what this formula means:
1: This represents the number of atoms centered on the plane.
3: This represents the area of the hexagonal unit cell.
R^2: This is the square of the atomic radius.
This formula tells us how many atoms are packed into a given area on the (0001) plane.
For example, in titanium, which has an atomic radius of 0.1445 nm, the planar density of the (0001) plane is approximately 16.03 nm-2. This means that there are approximately 16.03 atoms packed into every square nanometer of the (0001) plane in titanium.
Planar density is a valuable concept for understanding the properties of materials. It can be used to predict things like the strength of materials, their ability to conduct electricity, and their susceptibility to corrosion. The higher the planar density, the more tightly packed the atoms are, leading to greater strength and other properties.
See more here: What Are The Properties Common To Both Hcp And Ccp? | Hcp And Ccp Have Same Density
Can HCP and CCP have the same density?
HCP has eight spheres per unit cell, while CCP has four. This difference in the number of atoms directly impacts the overall density. Think of it this way: if you have the same amount of material (the same element) but pack it into different containers (unit cells) with varying numbers of “items” (atoms), the density will be different.
Here’s a deeper look at why this is the case:
HCP (Hexagonal Close-Packed) Structure: This structure is characterized by a hexagonal arrangement of atoms. The layers stack in an ABABAB pattern, meaning that each layer is identical, but they’re shifted so that the atoms in one layer sit directly over the gaps in the layer below.
CCP (Cubic Close-Packed) Structure: This structure is characterized by a cubic arrangement of atoms. The layers stack in an ABCABC pattern, meaning that the third layer is shifted from the first and second, creating a different stacking arrangement.
This difference in stacking patterns, while seemingly subtle, has a major impact on the number of atoms that can be packed into a unit cell. HCP has a slightly higher packing efficiency than CCP, meaning more atoms can be packed into the same space. This higher packing efficiency leads to a higher density in HCP structures compared to CCP structures, even if they’re made up of the same element.
So, while HCP and CCP share a similar close-packed arrangement, their subtle differences in stacking lead to a distinct difference in density. This is a key point to remember when considering the properties of different crystalline materials.
What is the coordination number of HCP & CCP structures?
While they have the same coordination number, HCP and CCP structures can’t have the same density even if they’re made of the same element. This is because their unit cells, the smallest repeating units of the crystal structure, contain a different number of atoms.
To understand this better, let’s look at how the HCP and CCP structures are arranged.
In the HCP structure:
* Atoms are arranged in a hexagonal pattern, forming layers that stack on top of each other in an ABABAB… sequence.
* The first layer (A) is directly above the third (A), forming a repeating pattern.
In the CCP structure:
* Atoms are arranged in a cubic pattern, forming layers that stack on top of each other in an ABCABC… sequence.
* The first layer (A) is directly above the fourth layer (A), creating a different repeating pattern.
This difference in stacking sequence affects the number of atoms in the unit cell. The HCP structure has two atoms per unit cell, while the CCP structure has four. Since density is calculated by dividing mass by volume, and the mass of the unit cell is directly proportional to the number of atoms, HCP and CCP structures of the same element will have different densities.
To visualize this, imagine stacking oranges. In HCP, you would stack them in a pattern where the oranges in the second layer sit in the depressions formed by the oranges in the first layer. In CCP, you would stack them in a pattern where the oranges in the second layer sit directly on top of the oranges in the first layer. The difference in stacking will affect how tightly the oranges are packed.
What is the packing fraction of HCP & CCP?
While both HCP and CCP structures have a packing efficiency of 74%, they don’t have the same number of atoms per unit cell. HCP has 6 atoms per unit cell, and CCP has 4. This difference in the number of atoms per unit cell means that the packing fraction, which is the ratio of the volume occupied by atoms to the total volume of the unit cell, is slightly different for the two structures.
To calculate the packing fraction, we need to know the volume occupied by the atoms and the total volume of the unit cell. Let’s break it down:
Volume occupied by atoms: For both HCP and CCP, the atoms are assumed to be hard spheres, and the volume occupied by an atom is given by (4/3)πr³, where r is the radius of the atom.
Total volume of the unit cell: This is a bit trickier. We need to consider the geometry of the unit cell and how the atoms are arranged.
For both HCP and CCP, the packing fraction can be calculated using the following formula:
Packing fraction = (Volume occupied by atoms) / (Total volume of the unit cell)
Since HCP has 6 atoms per unit cell and CCP has 4, the volume occupied by atoms will be different for the two structures. Additionally, the total volume of the unit cell will also differ due to the unique arrangements of atoms in each structure. This is why, even though the packing efficiency is the same, the packing fraction is slightly different for HCP and CCP.
While the packing fraction is not exactly the same for both structures, it is very close. Both structures achieve a very high packing efficiency of 74%, meaning that there is very little empty space within the structure.
Do all metals have a CCP or hcp structure?
While many metals *do* have either HCP, CCP, or BCC structures, some metals are a bit more flexible. These metals can switch between HCP and CCP structures depending on the temperature and pressure they’re experiencing. It’s like they have a built-in shape-shifting ability!
But let’s break down what we’re talking about here. CCP, HCP, and BCC are different ways that atoms can be arranged in a solid metal. They’re like different building blocks that make up the metal’s overall structure.
CCP (Cubic Close-Packed) is like a perfectly stacked box of oranges. The atoms are arranged in a cubic pattern, with each atom touching 12 other atoms.
HCP (Hexagonal Close-Packed) is like a honeycomb structure. The atoms are arranged in a hexagonal pattern, with each atom touching 12 other atoms.
BCC (Body-Centered Cubic) is like a cube with an atom in the center. The atoms are arranged in a cubic pattern, with each atom touching 8 other atoms.
Metals that can switch between HCP and CCP structures do so because the difference in energy between the two arrangements is small. This means that a little bit of extra heat or pressure can be enough to make the atoms rearrange themselves into a new structure.
For example, Cobalt can exist in either HCP or FCC structures. At low temperatures, it’s typically HCP, but when you heat it up, it transitions to FCC. This is because the FCC structure allows for more space between atoms, which is more favorable at higher temperatures.
So, to answer your question directly: No, not all metals have a CCP or HCP structure. Some metals can have different structures depending on conditions, like temperature and pressure. And it’s the subtle differences in energy that determine which structure a metal will prefer under different circumstances!
See more new information: bmxracingthailand.com
Hcp And Ccp: Why Do They Have The Same Density?
HCP and CCP: Close Cousins in the Crystal World
You might be wondering, what’s the big deal about HCP and CCP having the same density? Well, it’s a bit like finding out that two twins have the same birthday – it seems logical but actually hides some interesting details.
Both hexagonal close-packed (HCP) and cubic close-packed (CCP) structures are known for their high packing efficiency. This means that atoms are arranged in a way that maximizes the use of space. Think of it like stacking oranges in a grocery store – you want to get as many oranges in the box as possible!
But here’s the twist: even though they have this high packing efficiency, they have different arrangements of atoms. This is like having two twins with the same height but different hairstyles – their overall volume is the same, but their appearance is different.
Think of it this way:
HCP is like a neat stack of coins – you have layers of atoms arranged in a hexagonal pattern.
CCP is more like a 3D puzzle – you have layers of atoms arranged in a cubic pattern.
What Makes Them Look the Same?
So, how can two different structures have the same density? The answer lies in the atomic packing factor (APF). This number tells us how much of the available space in the crystal structure is actually occupied by atoms.
Both HCP and CCP structures have an APF of 0.74. This means that 74% of the space is filled by atoms. It’s like saying that if you have a box of oranges, 74% of the box is actually filled with orange flesh.
This high APF is what makes both HCP and CCP structures very strong and stable.
A Closer Look: HCP and CCP Differences
Even though they have the same density, HCP and CCP have some key differences:
Symmetry: HCP has hexagonal symmetry while CCP has cubic symmetry.
Coordination Number: Both HCP and CCP have a coordination number of 12 meaning that each atom has 12 nearest neighbors.
Stacking Sequence: This is where the real difference lies. HCP has an ABAB stacking sequence. This means that the second layer of atoms sits in the depressions of the first layer. The third layer aligns with the first layer, and so on.
CCP has an ABCABC stacking sequence. This means that the second layer of atoms sits in the depressions of the first layer. The third layer is shifted so that its atoms are not directly over any of the atoms in the first two layers. The fourth layer aligns with the first layer, and so on.
Density: More than Just Atoms
Density is a measure of how much mass is packed into a given volume. For HCP and CCP structures, we’re talking about the mass of the atoms within a specific volume of the crystal.
The density of a material can be calculated using the following formula:
Density = Mass/Volume
Let’s break down the components:
Mass: This is the total mass of the atoms within the crystal structure.
Volume: This is the total volume occupied by the crystal structure.
Both HCP and CCP structures have the same atomic packing factor. This means that for a given volume, they have the same number of atoms. Since the atoms are the same size and type, their mass is also the same.
Therefore, because the mass and volume of both HCP and CCP structures are the same, they have the same density.
Examples of HCP and CCP Structures
Here are some examples of elements that crystallize in HCP and CCP structures:
HCP: Magnesium (Mg), Zinc (Zn), Titanium (Ti), Cadmium (Cd)
CCP: Copper (Cu), Silver (Ag), Gold (Au), Aluminum (Al)
Conclusion: Why Does Density Matter?
The density of a material is an important property that affects its behavior and applications. For example, materials with high density are often used for construction and engineering applications, while materials with low density are used for packaging and insulation.
Understanding the factors that affect density is crucial for developing new materials with specific properties.
FAQ: Addressing Your Questions
1. Why does the packing efficiency matter?
Think of it like fitting as many people as possible into a room. The more efficient the packing, the more people you can fit in. A high packing efficiency means that the atoms are packed together as tightly as possible, leading to strong and stable structures.
2. Are there any other crystal structures with the same density as HCP and CCP?
Yes, there are! Face-centered tetragonal (FCT) structures can also have the same density as HCP and CCP. This is because the arrangement of atoms in FCT is slightly different, but it still results in a high packing efficiency.
3. Can the density of HCP and CCP change?
Yes! If you apply pressure to the crystal structure, the density can increase. This is because the pressure will force the atoms closer together.
4. Does the density of HCP and CCP change with temperature?
In general, the density of HCP and CCP structures decreases as temperature increases. This is because the atoms are vibrating more at higher temperatures, which increases the distance between them.
5. Why do elements form different crystal structures?
The crystal structure that an element adopts depends on a number of factors, including:
The size of the atoms
The type of bonding between the atoms
The temperature and pressure at which the element is formed
6. What are the practical implications of understanding HCP and CCP structures?
Knowing the crystal structures of different materials is crucial for a variety of fields, including:
Materials science: To design materials with specific properties, such as strength, ductility, and conductivity.
Metallurgy: To understand how metals deform and fracture under stress.
Ceramics: To develop ceramic materials with improved toughness and thermal resistance.
7. Can we predict the crystal structure of a new material?
Predicting the crystal structure of a new material is a complex process that requires extensive computational modeling and experimental verification. However, by understanding the fundamental principles of crystal structure, we can make informed predictions and design materials with specific properties.
Remember, even though HCP and CCP structures have the same density, they differ in their arrangements of atoms. This difference impacts their properties and applications. So, keep that in mind when working with these structures.
Closest Packed Structures – Chemistry LibreTexts
The hexagonal closest packed (hcp) has a coordination number of 12 and contains 6 atoms per unit cell. The face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. Chemistry LibreTexts
The hcp and ccp structures for a given element
The hcp and ccp structure cannot have the same density even if they both are made up of the same element because both these structures have different numbers of atoms per unit cell. The hcp Vedantu
7.8: Cubic Lattices and Close Packing – Chemistry LibreTexts
If they are the same, we have a body-centered cubic lattice. If they are different, and especially if they are oppositely-charged ions (as in the CsCl structure), Chemistry LibreTexts
12.2: The Arrangement of Atoms in Crystalline Solids
The hcp and ccp arrangements fill 74% of the available space and have a coordination number of 12 for each atom in the lattice, the number of nearest neighbors. Chemistry LibreTexts
Hexagonal Close Packing vs. Cubic Close Packing: What’s the
Key Differences. Hexagonal close packing (HCP) and cubic close packing (CCP) are both ways to efficiently arrange equal-sized spheres in a three-dimensional Difference Wiki
HEXAGONAL CLOSE-PACKED STRUCTURE – University of
A CCP crystal is a close-packed structure with the stacking sequence …ABCABC… To construct: 1st layer: 2D HCP array (layer A) 2nd layer: HCP layer with each sphere UCI Department of Chemistry
(IUCr) Close-packed structures
Elements in the same group tend to have the same structure at room temperature; for example, the alkali metals and Be, Mg, Zn and Cd (group IIA and IIB) are hcp; Cu, Ag and Au (group IB) are ccp. The elements International Union of Crystallography
Hexagonal Close Packing – Structure and HCP Structure Unit
Hexagonal close packing, or hcp in short, is one of the two lattice structures which are able to achieve the highest packing density of ~74%, the other being face Vedantu
The hcp and ccp structure for a given element would be expected
Solution. The correct option is C The same packing fraction. Both ccp and hcp have same co-ordination number = 12. In both unit cells, the packing efficiency is 74%. Density of BYJU’S
Hexagonal Close-Packed (HCP) Unit Cell | Materials
The HCP crystal structure is based on the Bravais lattice of the same name, with 1 atom per lattice point at each corner of the hexagonal prism, and 3 inside the prism. HCP is one of the most Materials Science & Engineering
Fcc Vs Hcp Stacking Example
Hexagonal Close Packed Crystal Structure
Hcp Vs Ccp
Explain Why Hcp And Ccp Structures For A Particular Solid Have Same Density.
Coordination Number Of Simple Cubic, Fcc, Bcc And Hcp Lattice
The Hcp And Ccp Structure For A Given Element Would Be Expected To Have: (A) The Same Co-Ordinat…
C To A Ratio For Hexagonal Close Packed (C/A=1.63)
An Hcp And A Ccp Structure For A Given Element Would Be Expected To Have\\N (A)The Sameco-Ordinat…
Link to this article: hcp and ccp have same density.
See more articles in the same category here: https://bmxracingthailand.com/what
