Let’s discuss the question: how many atoms are in 2.5 moles of zinc. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.

How many atoms are in 2.50 moles?
2.5mols of helium is present in 10g of helium. Explanation: 023×10^23 He atoms.
How many atoms are in a mole of zinc?
For the rest of the atoms on the periodic table, their molar mass tells you how many grams of each you need to have a mole of those atoms, or 6.02×1023 atoms of that element. If zinc has 1 mole, it will have 6.02×1023 .
Moles To Atoms Conversion – Chemistry
Images related to the topicMoles To Atoms Conversion – Chemistry

How many atoms are in 2.5 moles of aluminum?
How many atoms of aluminum is this? 2.5 mol 16.02×1023 atoms.
How many molecules are in 2.5 moles?
To get the number of molecules in 2.5 moles of CO2, you have to multiply the number of moles of CO2 by the Avogadro’ constant (6.022 X 10^23). Therefore, the number of molecules in 2.5 moles of CO2 would be: (6.022 X 10^23 X 2.5)=1.5055 X 10^24 molecules of carbon (IV) oxide.
How many moles of atoms are in 2.5 mol?
precisely. The mole is thus the link between the micro world of atoms, and molecules, to the macro world of grams and litres. 2.5⋅mol×6.022×1023⋅mol−1=15.06×1024⋅nickel atoms .
How many atoms are in 2.50 moles o2?
Therefore, a number of atoms present in 2.50 moles of oxygen are 3.01 ×10^24.
How many atoms are in zinc?
We know that one mole of zinc contains 6.022 × 1023 atoms of zinc. Therefore, 10 grams of zinc contain 9.264 × 1022 atoms.
How many atoms does zinc have?
| Group | 12 | 419.527°C, 787.149°F, 692.677 K |
|---|---|---|
| Period | 4 | 907°C, 1665°F, 1180 K |
| Block | d | 7.134 |
| Atomic number | 30 | 65.38 |
| State at 20°C | Solid | 64Zn |
What atoms are in zinc?
Zinc is the first element of the twelfth column of the periodic table. It is classified as a transition metal. Zinc atoms have 30 electrons and 30 protons with 34 neutrons in the most abundant isotope. Under standard conditions zinc is a hard and brittle metal with a bluish-white color.
How much atoms are in a mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
How many atoms are there in one mole of aluminium?
Finally, to convert the number of moles to atoms, use the fact that 1 mole of aluminium must contain 6.022⋅1023 atoms of aluminium → this is known as Avogadro’s constant.
How do you convert moles to molecules?
- To go from moles to molecules, multiply the number of moles by 6.02 x 1023.
- To go from molecules to moles, divide the numbers of molecules by 6.02 x 1023.
Converting Between Moles, Atoms, and Molecules
Images related to the topicConverting Between Moles, Atoms, and Molecules
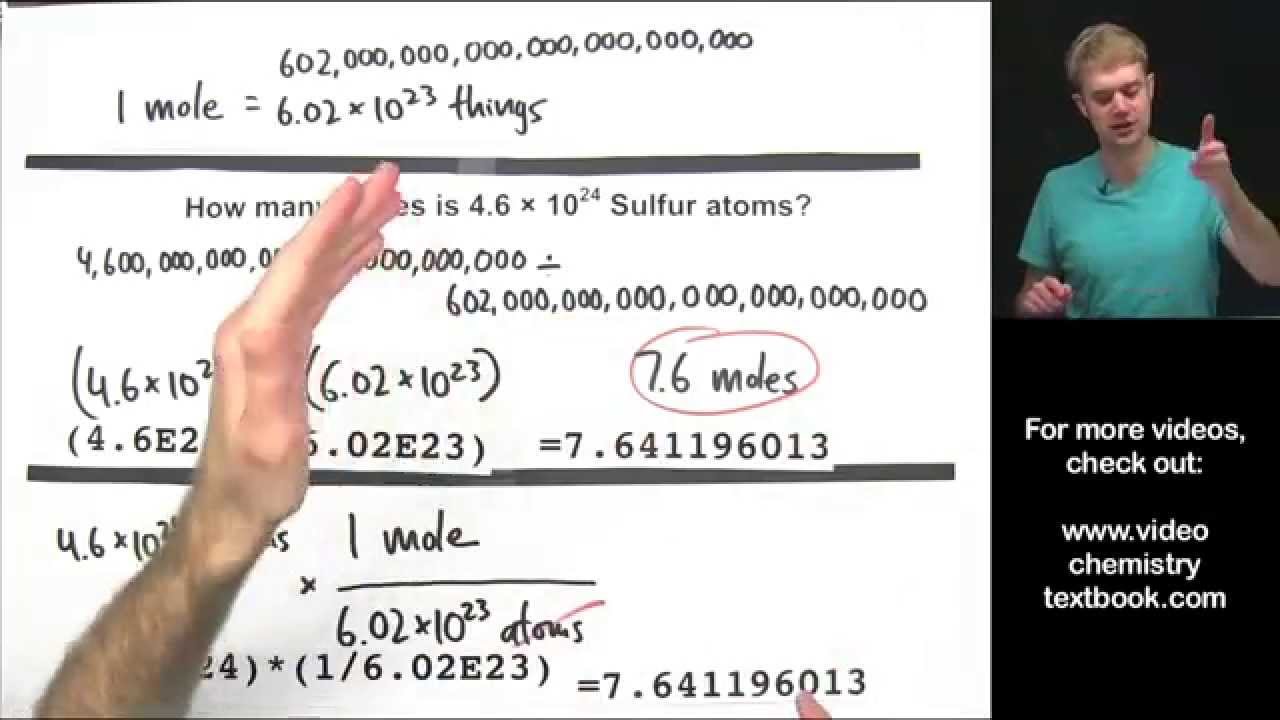
How many atoms are in 2.5 moles of h2o?
1 mole of water contains molecules of water. 2.50 moles of water will contain molecules of water. Each molecule of water has 2 atoms of hydrogen.
How many water molecules are in 2.5 moles?
1 Answer. Christopher A. Stefan V. 1.5×1024 molecules.
Which of the following is equal to 2.5 moles of CO2?
Mass of CO2 = number of moles x molecular mass of CO2.
Hence, the mass of 2.5 moles of CO2 is 110 grams.
How many atoms are in 2.5 moles of hydrogen?
How many atoms are there in 2.5 grams of hydrogen? So, in 2.5 moles of hydrogen, there are 2.5×2×6.022×1023 hydrogen atoms = 30.11×1023 hydrogen atoms.
How many atoms are in 2.50 moles of carbon dioxide molecules?
There are 1.51 x 1024 molecules of carbon dioxide in 2.50 moles of carbon dioxide.
How many atoms of oxygen are in 2.5 moles of SO2?
There are approximately 30.11 X 1023 atoms of oxygen in 2.50 moles of SO2.
How many atoms of sulfur are in 2.50 moles of SO2?
Answer and Explanation: There are 45.165 X 1023 atoms in 2.5 moles of SO2.
How many oxygen atoms are in 2.5 moles of c6h12o6?
The compound contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
How many moles of hydrogen atoms are there in 2.50 mol of c6h12o6?
Answer and Explanation: 1 mole of the compound, there 12 moles of Hydrogen atom.
How many atoms are in zinc sulphate?
The number of atoms of each element present is: Zinc (1 atom) Sulfur (1atom) Oxygen (4 atoms)
Number of Atoms in a Mole
Images related to the topicNumber of Atoms in a Mole

How many atoms are there in 2 moles of silver?
And, thus in 2.00⋅mol of silver, there are 2⋅mol×NA = 6.022×1023⋅mol−1 ×2⋅mol =1.20×1024 individual silver atoms. What is the mass of this number, this quantity of silver atoms?
How many neutrons are in zinc?
| PubChem CID | 91574 |
|---|---|
| Description | Zinc-65 atom is a zinc atom in which the nucleus contains 35 neutrons. It has a half-life of 244 days, decaying by emission of a positron (beta(+) decay), and is the most abundant and stable of the 25 known radioisotopes of zinc. ChEBI |
Related searches
- how many atoms are in 3.2 moles of zinc
- given 3 25 mol agno3 determine the number of formula units
- how many atoms are in a mole of zinc
- calculate the number of molecules in 11 5 mol of water
- how many moles are in 5.75 x10^24 atoms of aluminum
- how many atoms are in 3 moles of zinc
- how many atoms are in 4 moles of hydrogen gas
- calculate the number of molecules in 11.5 mol of water
- how many molecules are in 2.50 moles of h2o?
- how many atoms are in 0.50 moles of zinc
- calculate the number of moles of hydrogen atoms in 11 5 mol water
- how many molecules are in 2 50 moles of h2o
- how many atoms are in 4.25 moles of zn
- how many moles are in 5 75 x1024 atoms of aluminum
- how many moles contain each of the following
- given 3.25 mol agno3, determine the number of formula units
- how many atoms are in 2.5 moles
- how many atoms are in 2.50 moles of zinc *
- determine the number of atoms in the following a 2 50 mol zn b 1 50 gc
- how many atoms are in 2.5 moles of zinc
Information related to the topic how many atoms are in 2.5 moles of zinc
Here are the search results of the thread how many atoms are in 2.5 moles of zinc from Bing. You can read more if you want.
You have just come across an article on the topic how many atoms are in 2.5 moles of zinc. If you found this article useful, please share it. Thank you very much.
