Let’s discuss the question: how many molecules are in 5.0 mg of aspartame. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.
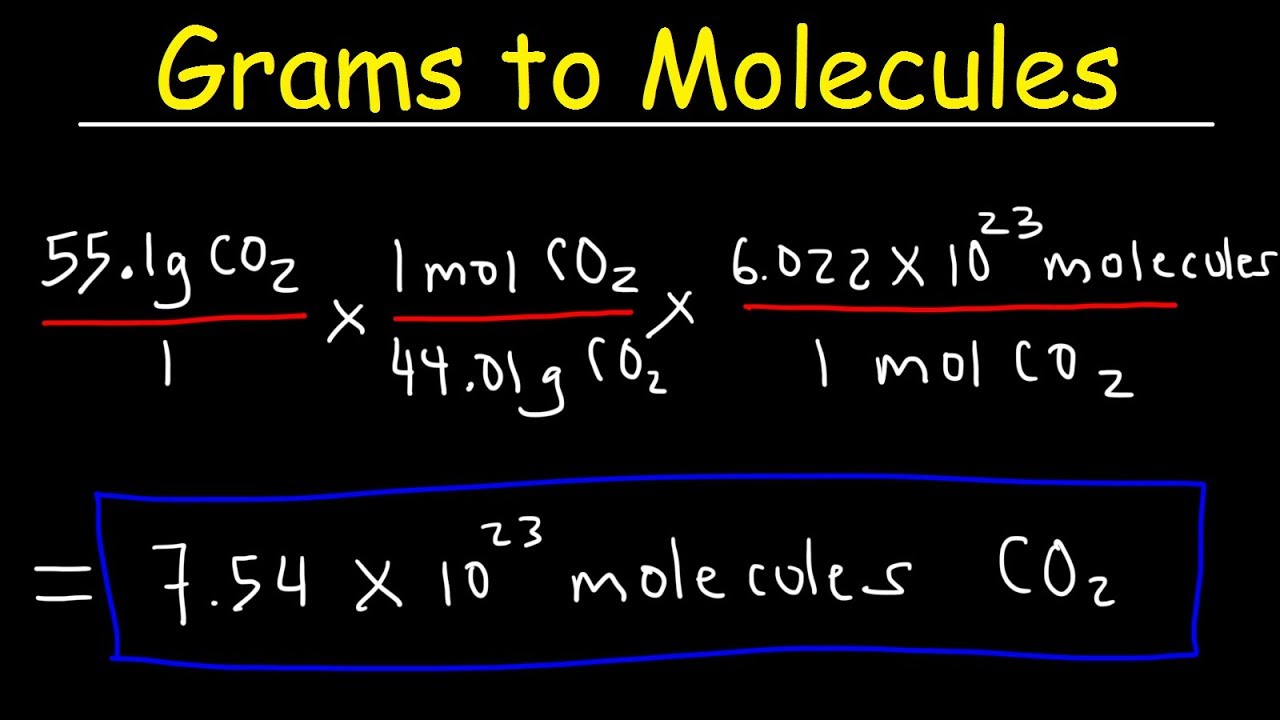
Table of Contents
How many molecules of Aspartame are present in 3.50 mg of Aspartame?
How many moles of aspartame are present in 3.50 mg of aspartame? The number of moles of aspartame are present in 3.50 mg of aspartame is 1.19×10–5 mol C14H18N2O5.
What is the mass in grams of 1 molecule of Aspartame?
Thus, 1 molecule of Aspartame weighs 4.88 × 10⁻²² g.
Grams to Molecules and Molecules to Grams Conversion
Images related to the topicGrams to Molecules and Molecules to Grams Conversion

How do you convert moles to molecules?
- To go from moles to molecules, multiply the number of moles by 6.02 x 1023.
- To go from molecules to moles, divide the numbers of molecules by 6.02 x 1023.
How many moles of Aspartame are present in 225 g of Aspartame?
The artificial sweetener aspartame formula C14H18N2O5 is used to sweeten foods. How many moles of aspartame are present in 225 g of aspartame. =0.765 moles.
How many hydrogen atoms are present in 1mg of Aspartame?
Therefore for every 1 mole of Aspartame we have 18 moles of Hydrogen atoms.
What is the mass of 1.00 mole of aspartame?
A. Calculate the molar mass of aspartame. 294.3 g/mol b.
How do you convert grams to moles calculator?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
How do you find the mass of one mole of aspartame?
The molar mass of a compound can be calculated by adding the standard atomic masses (in g/mol) of the constituent atoms. How many moles are in 5 mg of aspartame? Mass of aspartame = 5 mg = 0.005 grams. Also, molar mass of aspartame = 294.3 grams.
How do you calculate molecules?
Explanation: Determine the mass of the substance, and its molar mass. Divide the given mass by its molar mass to get moles, then multiply times 6.022×1023molecules1mol .
How many molecules are in a gram?
The definition of Avogadro’s number of 6.022 × 1023/mole is the number of atoms or molecules per one gram atomic weight.
Calculate the Mass of a Single Atom or Molecule
Images related to the topicCalculate the Mass of a Single Atom or Molecule

How many molecules are in 1.5 moles?
Hence, number of molecules in 1.5 moles of ammonia is 9.033 × 1023.
How many molecules are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
How many grams are in 4.5 moles of CO2?
Selina – Chemistry – Class 7
* The molar mass of CO2 is calculated as: Sum of atomic mass of carbon and oxygen. Hence, mass of 4.5 moles of CO2 = 198u.
What is the mass of 5 moles of Fe2O3?
2. What is the mass of 5 moles of Fe2O3 ? Smal x 159.0 1798.45g Page 2 3.
How many moles of O2 react with 4.5 moles of NH3?
The reaction above can mean: 4 molecules of NH3 reacts with 5 molecules of O2 to produce 4 molecules of NO and 6 molecules of H2O. It also can be interpreted as: 4 moles of NH3 reacts with 5 moles of O2 to produce 4 moles of NO and 6 moles of H2O.
How many atoms are there in aspartame?
| Property Name | Property Value | Reference |
|---|---|---|
| Heavy Atom Count | 21 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 380 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
What is the chemical formula for aspartame?
How many moles of nitrogen are in aspartame?
For mol aspartame → mol nitrogen, there are 2 moles of nitrogen present in mole of aspartame as per its chemical formula.
How do I calculate molar mass?
Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole.
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

What is the molar mass of 1.56 mol of aspartame c14h18n2o5?
The molar mass Was the 1 41.94.
What is the percent composition of aspartame?
C=57.14%H=6.16%N=9.52%O=27.18%
Related searches
- how many atoms of nitrogen are in 1 2000 grams of aspartame
- how many atoms of nitrogen are in 1.2 grams of aspartame
- how many molecules are in 5 mg of aspartame brainly
- how many hydrogen atoms are present in 4 50 mg of aspartame
- how many atoms of nitrogen are in 1.2000 grams of aspartame?
- how many atoms are in 1 2 g of aspartame
- how many hydrogen atoms are in 1 mg of aspartame
- how many hydrogen atoms are present in 4.50 mg of aspartame?
- how many hydrogen atoms are present in 5 00 mg of aspartame
- how many atoms of nitrogen are in 1 2 grams of aspartame
- how many moles of aspartame are present in 1 mg of aspartame
- how many hydrogen atoms are present in 5.00 mg of aspartame?
Information related to the topic how many molecules are in 5.0 mg of aspartame
Here are the search results of the thread how many molecules are in 5.0 mg of aspartame from Bing. You can read more if you want.
You have just come across an article on the topic how many molecules are in 5.0 mg of aspartame. If you found this article useful, please share it. Thank you very much.
