Let’s discuss the question: how many moles in hcl. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.
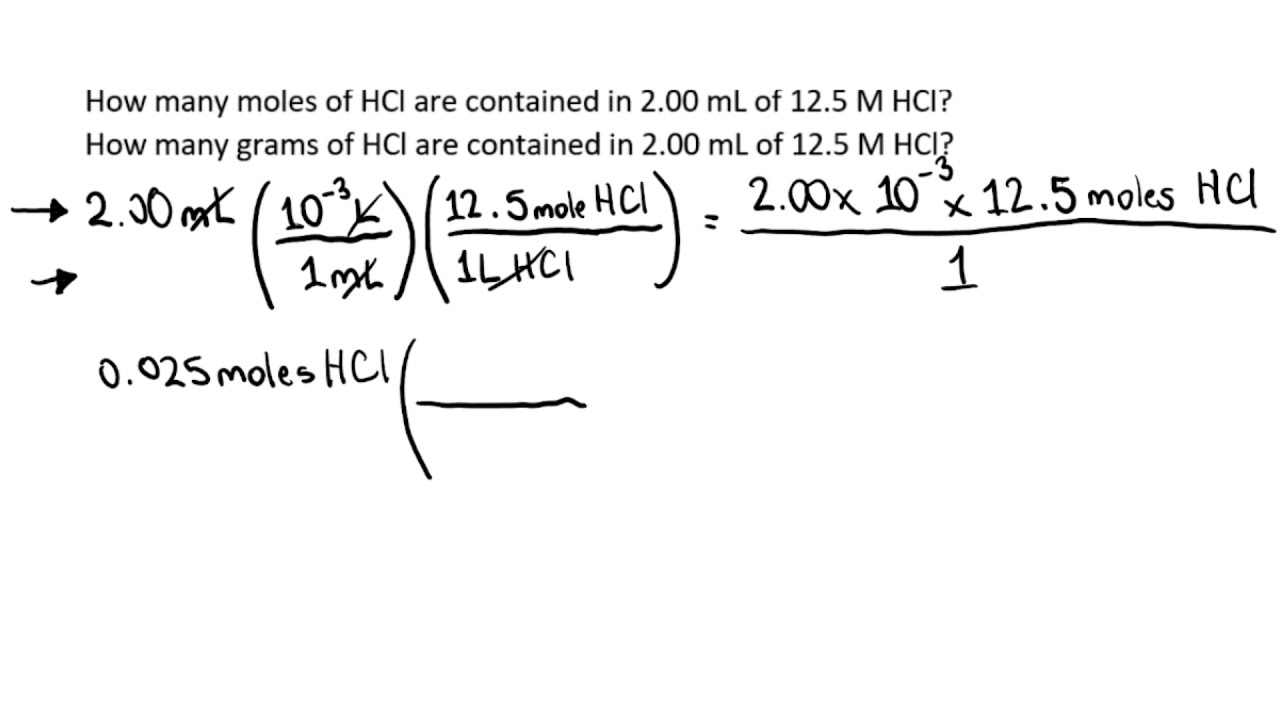
Table of Contents
How do you calculate moles of HCl?
Calculate the molarity of an HCl solution which contains 18.23 g of HCl in 355.0 mL of solution. Calculate the number of moles of HCl. Divide the number of moles of HCl by the total volume in liters.
How many mole does HCl have?
Given: The number of moles of HCl = 0.1. The number of particles in one mole = 6.022 × 1023. The mass of one mole of any substance is its molar mass, so the mass of one mole of HCl = 1 + 35.5 = 36.5g.
General Chemistry: How many moles and grams of 12 5 M HCl
Images related to the topicGeneral Chemistry: How many moles and grams of 12 5 M HCl

How many moles are in a gram of HCl?
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles HCl, or 36.46094 grams.
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
How do you make 2 molar HCl?
- 1M HCl: add 1mol/12M = 83 ml conc. HCl to 1L of water or 8.3ml to 100ml.
- 2M HCl: add 2mol/12M = 167 ml conc. HCl to 1L of water or 16.7ml to 100ml.
How many moles are?
…
Applications of the Mole.
| Known Information | Multiply By | Result |
|---|---|---|
| Moles of substance (mol) | Avogadro’s constant (atoms/mol) | Atoms (or molecules) |
How many moles are there in 73g HCl?
Best Answer
(a) No. of molecules in 73 g HCl = 6.023 x1023 x 73/36.5(mol.
What is the volume of HCl?
Concentrated hydrochloric acid has concentration of 12.19 mol/litre, The molar mass of HCl is 36.458 g/mol. 125 g is therefore (125/36.458) = 3.4286 moles. So the volume that would contain this number of moles is going to be (3.4286 / 12.19) = 0.28126 litres or 281.26 ml.
How do you find the concentration of HCl?
- Concentration in mol/dm 3 =
- Concentration in mol/dm 3 =
- = 0.125 mol/dm 3
- Relative formula mass of HCl = 1 + 35.5 = 36.5.
- Mass = relative formula mass × amount.
- Mass of HCl = 36.5 × 0.125.
- = 4.56 g.
- So concentration = 4.56 g/dm 3
What is the mass of 2 moles of HCl?
2 moles HCl to grams = 72.92188 grams.
What is molecular weight of HCl?
How many moles of HCl are present in 1 litre of 1 M HCl solution ?
Images related to the topicHow many moles of HCl are present in 1 litre of 1 M HCl solution ?

What is in hydrochloric acid?
Hydrochloric acid (CASRN 7647-01-0) is used then released via effluent flows by the paper industry. It is a solution of hydrogen chloride (HCl) dissolved in water. HCl is a highly corrosive, strong acid, and can be a clear/colorless or light yellow liquid.
What is a 1 mole?
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
What are moles in chemistry?
A mole is a very important unit of measurement that chemists use. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs means you have twelve eggs. Chemists have to measure using moles for very small things like atoms, molecules, or other particles.
What does n m/m mean?
n = m/M n is the amount of substance, in moles, mol. m is the mass of the substance, in grams, g. M is the molar mass of the substance (the mass of one mole of the substance) in g mol-1. Molar masses: These. will be given in.
What is 2M HCl?
2M HCl: add 2mol/12M = 167 ml conc. HCl to 1L of water or 16.7ml to 100ml.
How do you make 1 molar HCl from 37?
Calculations: Stock bottle of 37% HCL. 37 ml of solute/100 ml of solution. Therefore add 8.3 ml of 37% HCL to 1 liter of D5W or NS to create a 0.1N HCL solution.
How many moles are in 1kg?
The idea here is that 1 kg-mole is equal to 103 moles. This is the case because a mole of a substance must contain a number of particles of that substance equal to the number of atoms present in exactly 12 g of carbon-12.
What is the mol of NaOH?
One mole of NaOH has a molar mass of 40.0 g, so 40.0 g of NaOH dissolved in 1 kg of water results in a one-molal NaOH solution. If 20.0 g of NaOH, which is 0.500 mol of NaOH, is dissolved in exactly 1 kg of water, the concentration of the solution is 0.500 m NaOH.
How do you find moles from liters?
At standard temperature and pressure [STP], 1 mole of ideal gas is equal to 22.4 liters. Thus, the conversion ratio used in the formula below is 22.4. Thus, the amount of substance in moles is equal to the volume of ideal gas in liters divided by the conversion ratio of 22.4 L/mol.
how many moles of HCl are present in 0.098 g of HCl?
Images related to the topichow many moles of HCl are present in 0.098 g of HCl?
How many ml of HCl is present in 1.5 mole of it?
1.5M HCL MEANS 1.5 MOLE OF HCL PRESENT IN 1 LITRE OR 1000 ML OF SOLUTION.
How many moles of HCl will be present in 100 ml?
6mole.
Related searches
- naoh grams to moles
- 1 mole of hcl
- how many moles are in 72.9 g of hcl
- how many moles are there in 73g hcl
- grams of hcl
- how many moles are present in a sample of hcl with a mass of 3.65 g
- 1 mole of hcl in grams
- how many moles are in 72 9 g of hcl
- 1 mole of hcl in ml
- how many moles of hcl will be present in 100 ml
- how many moles of hcl are present in the original 25.00 ml of acid
- how many moles of hcl are present in 0.098 grams of hcl
- how many moles of hcl are consumed in the production of 7.5 moles of mgcl2
- molar mass of hcl
- moles of hcl calculator
- how many moles of hcl are present in 0.70
- how many moles of hcl are in the 20.ml sample of 0.10m hcl(aq)
Information related to the topic how many moles in hcl
Here are the search results of the thread how many moles in hcl from Bing. You can read more if you want.
You have just come across an article on the topic how many moles in hcl. If you found this article useful, please share it. Thank you very much.
