Let’s discuss the question: how many moles of iodide ions reacted. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.

Table of Contents
How many moles are in iodine?
…
Atoms in the RDA of Iodine.
| Molecule | Molecular Weight | Mass of 1 Mol of Molecules |
|---|---|---|
| Br2 | 2(79.90) = 159.80 | 159.80 g |
| O2 | 2(16.00) = 32.00 | 32.00 g |
| H2O | 2(1.008) + 16 = 18.02 | 18.02 g |
How many moles of I2 are liberated?
Hence, 3 moles of iodine will be liberated.
Stoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems
Images related to the topicStoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems

What is the mole ratio of zinc iodide?
SInce you can’t have a whole number that’s smaller than 1 (and different from zero, of course), it follows that 1:2 is the smallest whole number ratio in which the two elements can be combined. You can double-check the result by using the molar mass of zinc iodide, ZnI2 , and the number of moles you have.
What is the reaction between zinc and iodine?
The reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed I2, and electrolysis. Mixture of Zn + I2 before water is added. Iodine vapor is produced when water is added. ZnI2 is formed.
How many moles of iodine are liberated when two moles of potassium permanganate react with potassium iodide?
According to above reaction 2 moles of KMnO4 gives 5 mole I2.
How many moles of KMnO4 are required in acidic medium?
The oxidation of ferrous ions to ferric ions involves one mole of electrons. The oxidation of oxalate ions to carbon dioxide involves 2 moles of electrons. In an acidic medium, one mole of KMnO4 requires 5 moles of electrons.
How do you find the moles of reacted zinc?
Write the equation of the chemical reaction, as follows: Zn + S = ZnS. Convert the mass of Zn to moles of Z by dividing the molar mass of Zn into the mass of Zn, as follows: 12 g Zn x 1 mole Zn/65.38 g Zn = 0.184 moles Zn.
How do you find moles of reacted zinc?
- Determine moles I2 by dividing the given mass by its molar mass. …
- Calculate moles Zn by multiplying moles I2 by the mole ratio between I2 and Zn in the balanced equation, with Zn in the numerator.
- Determine the mass of the Zn required to react with 12.5 g I2 by multiplying the moles Zn by it molar mass.
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
Calculating Moles of Ions II (Pre-Name Change)
Images related to the topicCalculating Moles of Ions II (Pre-Name Change)

What is the molar mass of iodine?
When Zn and I2 reacts to form znl2 the one that is oxidised?
So, in this reaction, the Zn metal has given two electrons which are taken by iodine atoms, one electron by each I atom. Thus, the Zinc metal is oxidised while the Iodine is reduced.
What is the formula for zinc iodide?
How many moles of KMnO4 are reacted with one mole of ferrous oxalate HD medium?
6 mole ofKMnO4is needed to react completely(oxidise completely) one mole of ferrous oxalate.
How many moles of FeSO4 react with one mole of KMnO4?
∴ 1 mol of KMnO4 will react with 5 moles of FeSO4.
How many moles of KMnO4 are required to oxidise ferric?
Thus, 6 moles of KMnO₄ are required in acidic medium to oxidise 10 moles of ferric oxalate.
How do you find the number of moles produced in a reaction?
- Step 1: Balance the Chemical Reaction.
- Step 2: Take the ratio of the product’s stoichiometric coefficient and the reactant’s stoichiometric coefficients.
- Step 3: Multiply the ratio obtained in Step 2 with the given number of moles of the reactant.
How many moles of lead (II) choride will be formed from a reaction between 6.5 g of PbO and 3.2 g of
Images related to the topicHow many moles of lead (II) choride will be formed from a reaction between 6.5 g of PbO and 3.2 g of
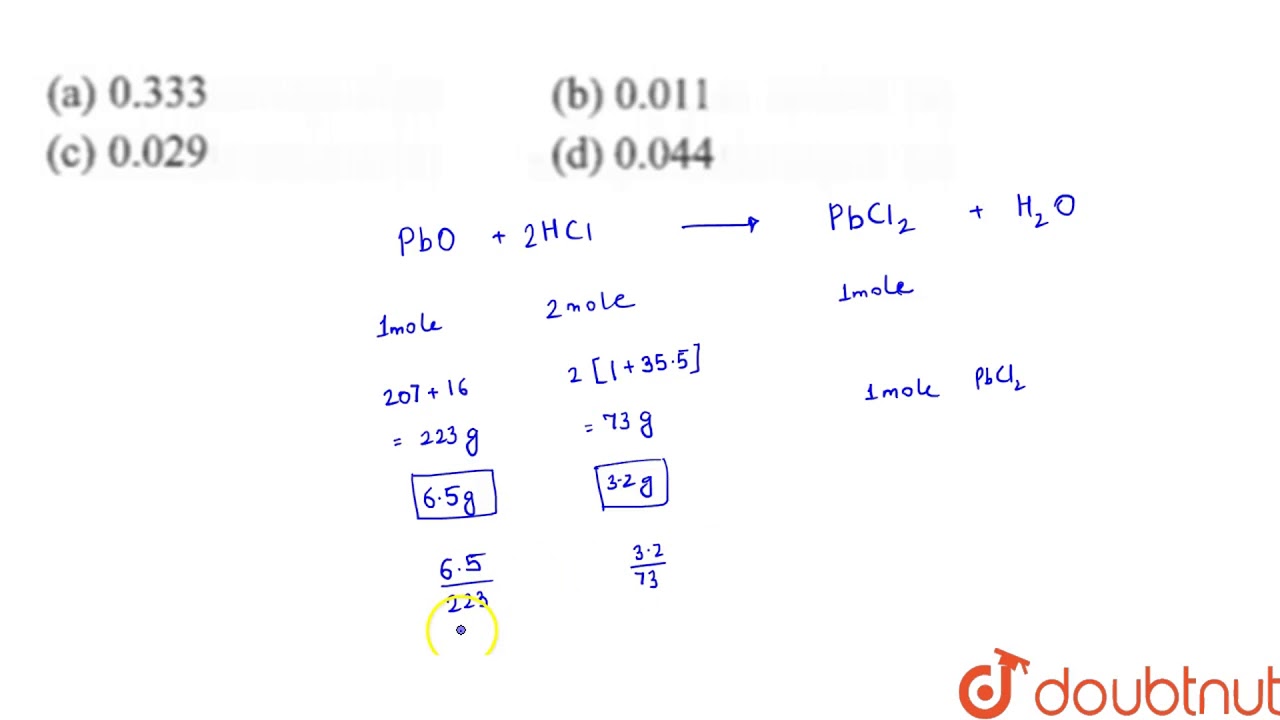
How do you find the number of moles needed to react?
Determine the number of moles needed to react by multiplying by moles of the known substance by the stoichiometric ratio of the unknown substance to the known substance.
How many moles are in Zn?
Zinc is a chemical element that you can find in the periodic table. The symbol for zinc is Zn, and its atomic number is 30. More importantly for the purposes of making our converter, the atomic mass of zinc is 65.38. That means that one mole of zinc weighs 65.38 grams (65.38 g/mol).
Related searches
- how many moles of iodide ions reaction
- what type of compound is zinc iodide?
- how to calculate number of moles that reacted
- what type of compound is zinc iodide
- zinc iodide molar mass
- how many moles reacted
- what type of structure is zinc iodide
- how many moles are produced in a reaction
- zinc iodine reaction
- empirical formula of zinc iodide experiment
- how to calculate moles of zinc reacted
- zinc iodide formula
- what is the limiting reactant in zinc iodide
Information related to the topic how many moles of iodide ions reacted
Here are the search results of the thread how many moles of iodide ions reacted from Bing. You can read more if you want.
You have just come across an article on the topic how many moles of iodide ions reacted. If you found this article useful, please share it. Thank you very much.
