Let’s discuss the question: how many moles of na2s2o3. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.
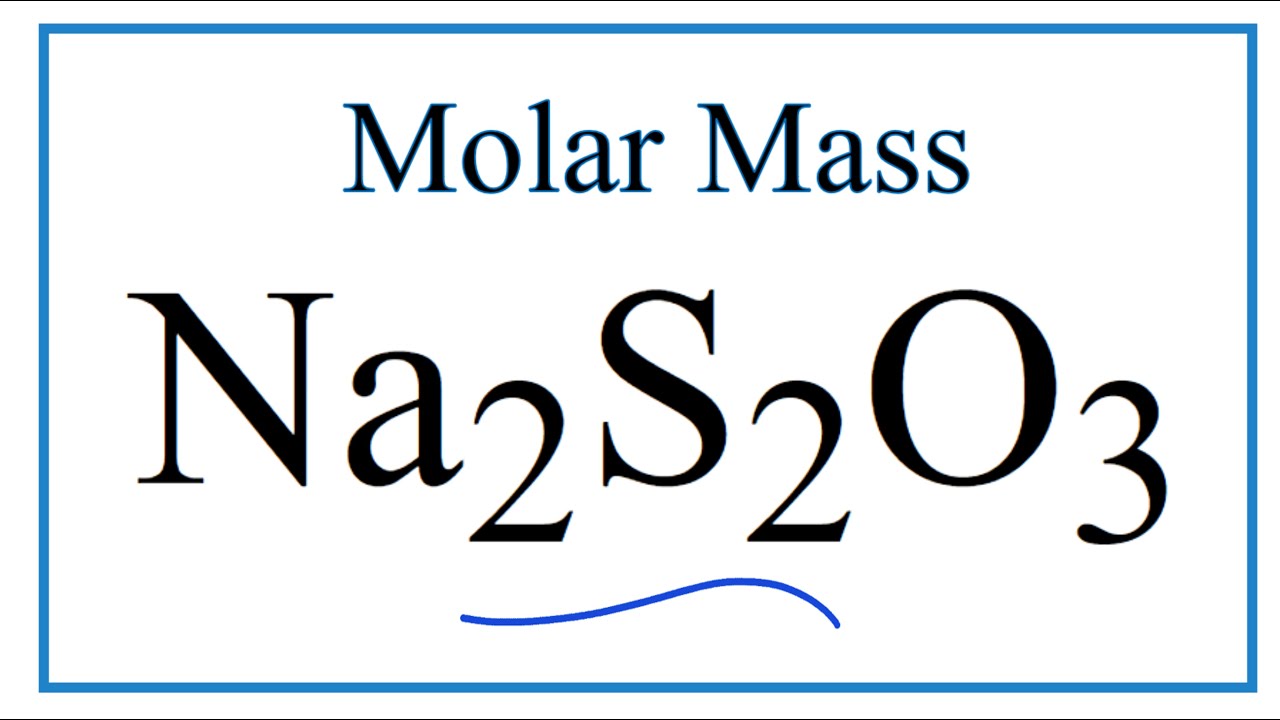
What are the moles of Na2S2O3?
To calculate the molar mass of sodium thiosulfate, Na2S2O3 , we need to find all the molar masses of the elements, and add them together. Na has a molar mass of 23 g/mol . So, Na2 will have a molar mass of 23 g/mol⋅2=46 g/mol .
How many moles are in thiosulfate?
The SI base unit for amount of substance is the mole. 1 grams Sodium Thiosulfate is equal to 0.0063248010502206 mole.
Molar Mass / Molecular Weight of Na2S2O3: Sodium thiosulfate
Images related to the topicMolar Mass / Molecular Weight of Na2S2O3: Sodium thiosulfate
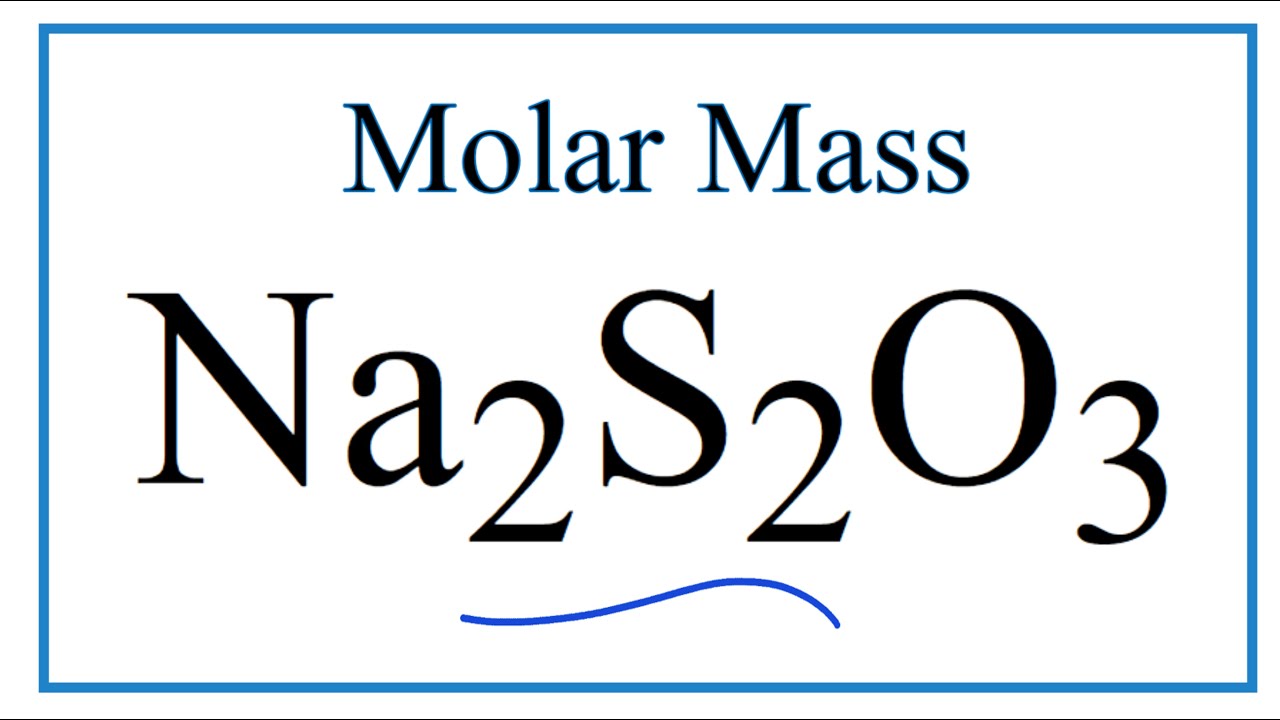
What is the molar mass of Na2S2O3?
How many grams are in Na2S2O3?
| PubChem CID | 24477 |
|---|---|
| Molecular Formula | Na2O3S2 or Na2S2O3 |
| Synonyms | SODIUM THIOSULFATE 7772-98-7 Sodium thiosulphate sodiumthiosulfate Disodium thiosulfate More… |
| Molecular Weight | 158.11 |
| Component Compounds | CID 24478 (Thiosulfuric acid) CID 5360545 (Sodium) |
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
What is the formula for Na2S2O3?
What is the volume of Na2S2O3?
Titration Lab average volume of Na2S2O3= 24.95mL sodium thiosulfate sollution= 0.1001M Cu2+ + 3I- -> CuI(s) +I2 2S2O32- + I2 -> 2I- +S4O62- 1) average number of moles of Cu2+ in each of the 25.00mL sample of the pipetted.
How many moles are in s2o3 2?
Ans : 13.25 gm.
How many total atoms are in Na2S2O3?
| Symbol | # Atoms | Atomic # |
|---|---|---|
| Na | 2 | 11 |
| S | 2 | 16 |
| O | 3 | 8 |
| Total Mass |
How many atoms are present in the compound Na2S2O3?
| Element | Symbol | Number of Atoms |
|---|---|---|
| Natrium | Na | 2 |
| Sulfur | S | 2 |
| Oxygenium | O | 3 |
Stoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems
Images related to the topicStoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems

What is the equivalent weight of Na2S2O3?
The equivalent weight of sodium thiosulphate = molecular weight of sodium thiosulphate. Therefore, the equivalent weight of sodium thiosulphate is 248.
What is the molar mass of sodium of sodium thiosulphate Na2S2O3 )?
How do I calculate molar mass?
Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole.
How do you convert grams to moles calculator?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
What is a 1 mole?
Originally, a mole was the quantity of anything that has the same number of particles found in 12.000 grams of carbon-12. That number of particles is Avogadro’s Number, which is roughly 6.02×1023. A mole of carbon atoms is 6.02×1023 carbon atoms. A mole of chemistry teachers is 6.02×1023 chemistry teachers.
How many moles are in NaCl?
One mol of NaCl (6.02 x1023 formulas) has a mass of 58.44 g.
How do you name Na2S2O3?
How do you prepare 0.01 N Na2S2O3?
Dissolve 24.8g of sodium thiosulphate pentahydrate(Na2S2O3. 5H2O) in 800 ml of freshly boiled and cooled water and mix thoroughly by shaking for approximately 15 minutes. Make up the volume to 1000 ml.
What is the name of Na2S4O6?
The chemical name of Na2S4O6 is sodium tetrathionate .
Oxidation Number of S in Na2S2O3 | HSC Chemistry 2 | Chapter 3: Quantitative Chemistry
Images related to the topicOxidation Number of S in Na2S2O3 | HSC Chemistry 2 | Chapter 3: Quantitative Chemistry

What is the density of sodium thiosulfate?
How is sodium thiosulfate formed?
Formation. Thiosulfate is produced by the reaction of sulfite ion with elemental sulfur, and by incomplete oxidation of sulfides (pyrite oxidation), sodium thiosulfate can be formed by disproportionation of sulfur dissolving in sodium hydroxide (similar to phosphorus).
Related searches
- molar mass of sodium thiosulfate pentahydrate
- how many moles of na2s2o3 can be produced from 32.07 g of sulfur
- na2s2o3 5h2o
- how many moles of na2s2o3 are needed to react with 0.12 mol of cl2
- how many moles are in 63 grams of na2co3
- how many moles of na2s2o3 are needed to react with 0.12mol of cl2
- grams to moles
- how many moles of na2co3
- how many moles of na2s2o3 are needed to react with 0.40 moles of cl2
- na2s2o3 structure
- how many moles of na2s2o3 are needed
- how many ml of 0.02 m na2s2o3 contain this number of moles
- how to calculate moles of na2s2o3
- moles of na2s2o3 used in titration
- molarity of na2s2o3
- molar mass of na2s2o3
- how many moles of na2s2o3 are needed to react with .12 mol of cl2
- how many moles are there in 17 3 g of sodium thiosulfate
- how many moles are there in 17.3 g of sodium thiosulfate
Information related to the topic how many moles of na2s2o3
Here are the search results of the thread how many moles of na2s2o3 from Bing. You can read more if you want.
You have just come across an article on the topic how many moles of na2s2o3. If you found this article useful, please share it. Thank you very much.
