What is the flow of electrons in a galvanic cell?
Think of it like this: The anode is where oxidation happens, meaning electrons are being lost. These lost electrons want to find a new home, and they do so by traveling through the external circuit to the cathode. At the cathode, reduction takes place, meaning the electrons are gained. This continuous flow of electrons from anode to cathode creates an electrical current.
Let’s delve deeper into the mechanics of this electron flow. Imagine you have a galvanic cell with a zinc electrode as the anode and a copper electrode as the cathode. The zinc electrode is immersed in a solution containing zinc ions (Zn²⁺), and the copper electrode is in a solution of copper ions (Cu²⁺).
Here’s what happens:
At the anode: Zinc atoms lose electrons and become zinc ions (Zn²⁺). These zinc ions move into the solution, while the electrons flow through the external circuit towards the cathode.
At the cathode: Copper ions (Cu²⁺) from the solution gain electrons and become copper atoms (Cu), plating onto the copper electrode.
The electrons flow continuously through the external circuit, driven by the difference in electrical potential between the two electrodes. This flow of electrons generates an electric current, which can be used to power devices.
Let’s be clear: The flow of electrons in a cathode ray tube is different from a galvanic cell. In a cathode ray tube, electrons are emitted from a heated cathode and accelerated towards a fluorescent screen. This is a completely different process than the electron flow in a galvanic cell.
Similarly, the flow of electrons in a thermionic diode is also distinct from a galvanic cell. A thermionic diode uses a heated cathode to emit electrons, which then travel to the anode. The direction of electron flow in this case is determined by the voltage applied to the anode and cathode, not by a chemical reaction as in a galvanic cell.
The key takeaway is that while the terms anode and cathode are used in all these devices, the mechanism of electron flow and the overall purpose are different.
Do electrons flow from anode or cathode?
Imagine a battery. It’s a chemical system that produces electricity through a spontaneous chemical reaction. In a battery, the anode is the negative terminal and the cathode is the positive terminal. The negative terminal has an excess of electrons, so they flow towards the positive terminal where there’s a deficiency of electrons.
Now, think about an electrolytic cell. It’s the opposite of a battery. Here, we use an external power source (like a battery charger) to force a non-spontaneous chemical reaction to occur. The external power source provides the energy needed to drive electrons from the anode to the cathode, even though the reaction itself is not naturally favorable.
This is a bit like pushing a rock uphill. You need to exert force to make it move upwards, even though gravity wants to pull it down. Similarly, in an electrolytic cell, the external power source provides the force needed to push electrons uphill, against the natural tendency of the reaction.
This external power source, essentially a battery charger, acts as a pump, moving electrons from the anode to the cathode. The anode becomes positively charged, as electrons are leaving, while the cathode becomes negatively charged due to the influx of electrons. This creates an electric potential difference that drives the chemical reaction.
So, in both batteries and electrolytic cells, electrons flow from the anode to the cathode. The difference lies in whether this flow is spontaneous (driven by a chemical reaction) or forced (driven by an external power source).
Where do ions flow in a voltaic cell?
Think of it like a game of tug-of-war. The electrons are the rope, and the ions are the teams pulling on opposite ends. The electrons flow from the anode to the cathode, and the ions flow in the opposite direction to keep the system balanced. This flow of ions is essential for maintaining the electrical neutrality of the cell and allows the chemical reactions to continue.
Let’s break down the flow of ions a little further. In a voltaic cell, the anode is where oxidation occurs, and the cathode is where reduction occurs. Oxidation is the loss of electrons, and reduction is the gain of electrons. When a metal anode is oxidized, it loses electrons and forms positive ions. These cations then move away from the anode into the electrolyte solution. Simultaneously, anions from the electrolyte solution move towards the anode to neutralize the positive charge buildup.
At the cathode, the opposite process occurs. Metal ions from the electrolyte solution gain electrons and are reduced to form a solid metal deposit. This process leaves behind a buildup of negative charge at the cathode. To balance this charge, cations from the electrolyte solution move towards the cathode.
This flow of ions through the electrolyte solution is crucial for the operation of a voltaic cell. It ensures that the cell remains electrically neutral and that the chemical reactions can continue. Without this flow of ions, the cell would quickly become unbalanced and stop functioning.
Where do the electrons flow from in a electrochemical cell?
Let’s break down why this happens. In an electrochemical cell, the anode is where oxidation occurs. Oxidation is the process of losing electrons. The cathode is where reduction occurs. Reduction is the process of gaining electrons. So, when a chemical reaction takes place in the electrochemical cell, the anode releases electrons, and these electrons then travel through the external circuit to the cathode, where they are accepted by the chemical species undergoing reduction.
Think of it this way: the anode is like a source of electrons, while the cathode is like a sink for electrons. The external circuit acts as the pathway for these electrons to travel from the source to the sink. This flow of electrons creates an electric current, which is what powers many electronic devices.
Here’s an analogy to help visualize this: Imagine a water wheel powered by a flowing river. The water flowing from the river represents the electrons flowing from the anode. The water wheel represents the cathode, where the water’s energy is used to do work. The river itself represents the external circuit, providing the pathway for the water to reach the wheel.
Where do the electrons flow in a voltaic cell from?
Think of it this way: The anode is where oxidation happens, meaning atoms lose electrons. These “lost” electrons are then attracted to the cathode, where reduction takes place and atoms gain electrons. So, the electrons essentially travel from the place where they’re being given up to the place where they’re being accepted.
Now, let’s consider the standard cell potential (Eocell) of the half reactions. The electrons will always flow from the half-reaction with the more negative standard cell potential to the half-reaction with the more positive standard cell potential. This is because the half-reaction with the more negative potential is more likely to lose electrons (oxidation), while the one with the more positive potential is more likely to gain electrons (reduction).
To visualize this, imagine a hill with the anode at the top and the cathode at the bottom. The electrons, like water flowing downhill, will naturally move from the higher energy state (anode, more negative potential) to the lower energy state (cathode, more positive potential).
A cell diagram is a simplified representation of an electrochemical cell. It shows the different components of the cell and the direction of electron flow. The cell diagram is a helpful tool for understanding how the cell works and for predicting the direction of electron flow.
Think of a voltaic cell like a battery: It provides a source of electrical energy through the controlled flow of electrons. This flow of electrons is what powers our devices and makes our world run.
Where do electrons flow in a cell?
In an electrolytic cell, electrical energy is converted into chemical energy. Electrons flow from the anode to the cathode through an external power supply. This is unlike a galvanic cell where the electrons flow from the anode to the cathode through an internal circuit.
Here’s the key thing to remember: electrons don’t actually flow through the electrolyte solution. Instead, it’s ions that move. Think of it this way: the anode is where oxidation happens, meaning it loses electrons. These electrons then travel through the external circuit to the cathode, where reduction happens, meaning they gain electrons. The ions in the electrolyte solution help to balance out the charges, allowing the chemical reactions to take place.
Let’s break down what’s happening with these ions in the solution:
– Anode: At the anode, positive ions are formed as the electrode loses electrons. These positive ions then move away from the anode and towards the cathode.
– Cathode: At the cathode, negative ions are formed as the electrode gains electrons. These negative ions then move away from the cathode and towards the anode.
This movement of ions in the electrolyte solution is crucial for the overall process of electrolysis. The electrons flowing through the external circuit are essentially just carrying the energy needed to drive the chemical reactions happening at the anode and cathode.
Why do electrons flow from Zn to Cu?
Zinc is more reactive than copper, meaning it’s more eager to give up its electrons. This difference in reactivity creates an electrical potential difference between the two metals.
Think of it like a seesaw: zinc is on the higher end, ready to release its electrons, while copper is on the lower end, ready to accept them. The electrons naturally flow from the higher potential (zinc) to the lower potential (copper) to try and balance things out.
This flow of electrons is what we call electricity. Since electrons are negatively charged, the zinc becomes the negative terminal of the battery, and the copper becomes the positive terminal.
Now, let’s delve a bit deeper into why zinc is so eager to lose electrons. It all comes down to the chemical makeup of the two metals.
Zinc has a weaker hold on its outermost electrons compared to copper. When zinc is placed in an electrolyte solution (like a weak acid or salt solution), it readily loses its electrons, forming positively charged zinc ions (Zn²⁺). These ions then dissolve into the solution, leaving behind the electrons on the zinc electrode.
These “orphaned” electrons are now looking for a place to go. Since copper is more electronegative, meaning it has a stronger attraction to electrons, it becomes the natural destination for these electrons. They flow through an external circuit (like a wire connecting the zinc and copper electrodes) to reach the copper electrode, completing the circuit.
This movement of electrons from zinc to copper generates an electrical current, which can be used to power devices.
What is the anode and cathode of a voltaic cell?
The anode is the electrode where oxidation happens. Oxidation is the loss of electrons. The cathode is the electrode where reduction happens. Reduction is the gain of electrons.
Think of it like this: The anode is where electrons are “given up,” and the cathode is where electrons are “taken in.”
To make this work, we need a salt bridge. The salt bridge is like a pathway for ions to move between the half-cells. This keeps the charges balanced and lets the chemical reaction keep going.
A voltaic cell is like a mini-battery. It uses chemical reactions to create an electric current. The anode and cathode are the key parts that make this possible.
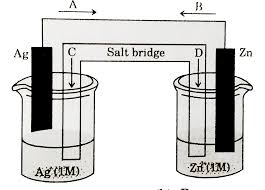
To get a better grasp of how the anode and cathode work, let’s take a look at a specific example: a voltaic cell made with copper and zinc.
In this voltaic cell, the zinc acts as the anode and the copper acts as the cathode. Zinc is more reactive than copper, so it will lose electrons more easily.
Here’s what happens:
* At the anode, zinc atoms lose electrons and become zinc ions (Zn2+). These ions go into solution. The electrons flow through the external circuit to the cathode.
* At the cathode, copper ions (Cu2+) in the solution gain electrons and become copper atoms. These copper atoms plate onto the copper electrode.
The flow of electrons from the anode to the cathode creates an electric current that can be used to power devices. This is how a voltaic cell generates electricity.
See more here: Do Electrons Flow From Anode Or Cathode? | In A Voltaic Cell Electrons Flow From The
How a voltaic cell is created?
Think of a voltaic cell as a tiny power plant, but instead of burning coal or oil, it harnesses the energy released when certain chemicals react. This reaction involves the transfer of electrons, which is what creates the electric current we can use.
To understand how a voltaic cell is created, we need to understand oxidation-reduction reactions. In these reactions, one substance loses electrons (oxidation) while another gains them (reduction).
When these reactions happen spontaneously, energy is released, and that’s where a voltaic cell comes in!
Here’s how a voltaic cell is created:
1. The Setup: You need two different electrodes (often metals) that are immersed in solutions containing their corresponding ions. For example, one electrode might be a copper strip in a solution of copper sulfate, and the other might be a zinc strip in a solution of zinc sulfate.
2. The Reaction: When the electrodes are connected through an external circuit, a chemical reaction occurs. The metal with a stronger tendency to lose electrons (in our example, zinc) will undergo oxidation, releasing electrons into the circuit. These electrons will flow through the external circuit towards the other electrode, which is the cathode. The electrons are then accepted by the metal ions in the solution surrounding the cathode (in this case, copper ions), causing them to be reduced.
3. The Flow of Electricity: The flow of electrons through the external circuit creates an electric current, which can be used to power devices.
4. The Salt Bridge: To complete the circuit, a salt bridge is used. This is a special device that allows ions to flow between the two solutions, maintaining electrical neutrality.
That’s essentially how a voltaic cell is created.
Think of the voltaic cell as a system where chemical energy is converted into electrical energy. By carefully selecting the materials and the solutions, you can create a voltaic cell that produces a specific voltage and current, providing a reliable source of energy!
What is the difference between Voltaic and electrolytic cells?
Voltaic cells, also known as galvanic cells, are like tiny batteries. They use chemical reactions to generate electrical energy. Think of them as nature’s power generators! On the other hand, electrolytic cells are the opposite. They use electrical energy from an external source to drive chemical reactions that wouldn’t happen spontaneously on their own. It’s like forcing a chemical reaction to happen!
Electrochemistry is all about the connection between electricity and chemical reactions. The key players in this game are oxidation-reduction (redox) reactions. In a redox reaction, electrons are transferred from one substance to another. This electron transfer is what creates the flow of electricity!
Let’s break down the differences between Voltaic and electrolytic cells a little further.
Voltaic cells harness the natural tendency of some chemical reactions to release energy in the form of electricity. They have a negative electrode, called the anode, where oxidation occurs (loss of electrons), and a positive electrode, called the cathode, where reduction occurs (gain of electrons). The flow of electrons from the anode to the cathode creates the electric current.
Electrolytic cells, on the other hand, use an external power source to force a non-spontaneous reaction to occur. They have a positive electrode, called the anode, where oxidation occurs, and a negative electrode, called the cathode, where reduction occurs. The external power source forces electrons to flow from the anode to the cathode, driving the chemical reaction forward.
In simpler terms, Voltaic cells are like batteries that produce electricity, while electrolytic cells are like reverse batteries that use electricity to drive chemical reactions.
Think about it this way: Imagine a hill with a ball at the top. If you let go, the ball will naturally roll down the hill. That’s like a Voltaic cell – the chemical reaction releases energy, just like the ball rolling downhill. Now, imagine pushing the ball back up the hill. That’s like an electrolytic cell – you’re using external energy to force the ball uphill, just like using electricity to drive a non-spontaneous chemical reaction.
What is voltaic cells?
Think of it like a game of hot potato, but with electrons instead of a spud! If this electron transfer happens naturally, meaning it wants to happen on its own, it releases energy. And guess what? That released energy can be used to do some pretty useful things!
So, voltaic cells are basically devices that harness this natural electron transfer, capturing the energy released and putting it to work. They’re like little energy generators powered by the simple exchange of electrons.
Now, let’s dive a little deeper into how this works. The voltaic cell is set up with two electrodes, usually made of different metals. These electrodes are immersed in solutions called electrolytes, which contain ions that can carry the electric charge.
One electrode, called the anode, is where oxidation happens. Oxidation is the process where a substance loses electrons. At the other electrode, the cathode, reduction takes place. Reduction means a substance gains electrons.
It’s like a dance: at the anode, electrons are “given away” and at the cathode, they are “accepted”. This movement of electrons creates an electric current that flows through an external circuit, powering whatever device is connected.
Think of it like a battery – that’s essentially what a voltaic cell is! The battery stores chemical energy, which gets converted into electrical energy when you use it. The chemical reaction driving the battery is a redox reaction, just like in a voltaic cell.
Voltaic cells are all around us, powering our everyday devices. They are the heart of batteries that run our phones, laptops, and cars. They are also used in many industrial processes to generate electricity.
So, the next time you use your phone or turn on your car, remember the tiny voltaic cells working hard to make it all possible!
What is a galvanic or voltaic cell?
Imagine a chemical reaction where one substance readily gives up electrons, while another eagerly accepts them. In a galvanic cell, we separate these two processes, creating a flow of electrons. These half reactions are the heart of the cell’s operation. One half reaction involves oxidation, where a substance loses electrons. This is the anode – where the electrons are released. The other half reaction is reduction, where a substance gains electrons. This happens at the cathode. The anode and cathode are connected by an external circuit, allowing the electrons to flow from the anode to the cathode. The difference in electrical potential between the anode and cathode creates the voltage that drives the current.
To complete the circuit, we need a salt bridge. This bridge allows ions to move between the two half-cells, maintaining electrical neutrality. It’s essential for preventing the build-up of charge that would stop the flow of electrons.
So, a galvanic cell is essentially a device that converts chemical energy into electrical energy. It’s like a tiny chemical factory that produces electricity through a controlled chemical reaction. The half reactions are the key to this process, driving the flow of electrons and generating a usable current.
See more new information: bmxracingthailand.com
In A Voltaic Cell Electrons Flow From The Anode To The Cathode.
In a voltaic cell, electrons flow from the anode to the cathode.
Let’s break down why this happens.
A voltaic cell is like a battery, generating electricity through a chemical reaction. It has two electrodes: the anode and the cathode. Each electrode is immersed in an electrolyte solution, which is a substance that conducts electricity.
Here’s the key:
The anode is where oxidation occurs. This means that atoms lose electrons. Think of it like this: The anode is giving up electrons.
The cathode is where reduction occurs. This means that atoms gain electrons. The cathode is accepting electrons.
So, since the anode is losing electrons and the cathode is gaining electrons, the electrons have to flow from the anode to the cathode.
Let’s use a simple example: a copper-zinc cell.
* The anode is made of zinc, which has a higher tendency to lose electrons than copper. When the zinc atoms lose electrons, they become zinc ions (Zn2+) and go into the solution.
* The cathode is made of copper, which has a lower tendency to lose electrons than zinc. The copper ions in the solution gain electrons and become copper atoms, plating onto the copper electrode.
The electrons released by the zinc anode flow through an external circuit, where they can power a device, and then reach the copper cathode. This flow of electrons creates an electric current.
Think of it like a waterfall:
* The anode is like the top of the waterfall, where the water is high and has a lot of potential energy.
* The cathode is like the bottom of the waterfall, where the water has less potential energy.
* The electrons are like the water, flowing from a higher energy state (anode) to a lower energy state (cathode) to even out the energy difference.
Here’s a simple analogy to remember:
Imagine a “Push and Pull” situation:
Push: The anode “pushes” electrons away because it is losing them.
Pull: The cathode “pulls” electrons towards itself because it is gaining them.
A quick recap:
Electrons flow from the anode to the cathode in a voltaic cell.
This flow is driven by the difference in the tendency of the two metals to lose electrons.
The anode is where oxidation occurs, and the cathode is where reduction occurs.
FAQs
Q: How does the electrolyte solution work?
A: The electrolyte solution allows the ions to move freely, completing the circuit. It’s like a bridge for the ions to travel from one electrode to the other.
Q: Why is the anode negative and the cathode positive?
A: The anode is considered negative because it is losing electrons and has a buildup of positively charged ions. The cathode is considered positive because it is gaining electrons and has a buildup of negatively charged ions.
Q: Can I make my own voltaic cell?
A: You sure can! You can create a simple voltaic cell using two different metal strips (like zinc and copper), some acidic solution (like vinegar), and some wires.
Q: How do I know which metal is the anode and which is the cathode?
A: You can use the Electrochemical Series to determine the relative tendency of different metals to lose electrons. Metals higher on the series have a higher tendency to lose electrons and will be the anode.
Let me know if you have any other questions. I’m always here to help!
20.3: Voltaic Cells – Chemistry LibreTexts
In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; only a single compartment is employed in most Chemistry LibreTexts
Voltaic Cells – Chemistry LibreTexts
Electrons always flow from the anode to the cathode or from the oxidation half cell to the reduction half cell. In terms of E o cell of the half reactions, the electrons will flow from the more negative half Chemistry LibreTexts
Galvanic (voltaic) cells (video) | Khan Academy
Galvanic (or voltaic) cells use a thermodynamically favored redox reaction to generate an electric current. Each half-reaction takes place in a separate compartment, or half-cell, containing an electrode. The electrode where oxidation occurs is the anode, and Khan Academy
How Voltaic Cells Function – University of Wisconsin–Madison
By separating the reactants into different containers, we can force the electrons to flow through an external circuit before returning to the reaction to carry out the reduction. Netorial
Galvanic Cells & Voltaic Cells | Electrochemical Cells – ChemTalk
In an electrochemical process, electrons flow from one substance to another substance in what is known as an oxidation-reduction (redox) reaction. Redox reactions happen ChemTalk
Introduction to galvanic/voltaic cells (video) | Khan Academy
How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current.. Shows the flow of electrons and ions, and explains the role of the salt Khan Academy
17.2 Galvanic Cells – Chemistry 2e | OpenStax
Devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place are called galvanic cells (or voltaic cells). A OpenStax
What is the flow of electrons, cations, and anions in a
The idea of the salt bridge is to prevent electrolytes mixing while providing ion flow. When you have a high concentration of inert ions in the salt bridge, cations in the salt bridge will flow into B, and anions in Chemistry Stack Exchange
Voltaic Cell | How Does It Work?
9.2 Describe How Current Is Conducted In An Electrolytic Cell [Sl Ib Chemistry]
Introduction To Galvanic Cells \U0026 Voltaic Cells
Working Of Voltaic Cell Or Simple Cell
Galvanic Cells (Voltaic Cells)
9.2 Voltaic Cells (Sl)
In The Electrolytic Cell, Flow Of Electrons Is From (A) Cathode To Anode Through Internal Supply….
In The Galvanic Cell, Flow Of Electrons Is From
Link to this article: in a voltaic cell electrons flow from the.
See more articles in the same category here: https://bmxracingthailand.com/what
