Is nh4cl an ionic or covalent compound?
The key here is the presence of a metal (in this case, ammonium (NH4+)) and a non-metal (chlorine (Cl-)). This combination is a hallmark of ionic compounds.
Ionic compounds are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions).
Ammonium (NH4+) is a polyatomic ion, meaning it’s a group of atoms that act as a single charged entity. It forms when a hydrogen atom (H+) bonds with a nitrogen atom (N) and three hydrogen atoms (H).
Chlorine, on the other hand, is a non-metal and gains an electron to form a negatively charged chloride ion (Cl-).
The electrostatic attraction between the positively charged ammonium ion (NH4+) and the negatively charged chloride ion (Cl-) leads to the formation of the ionic compound, ammonium chloride (NH4Cl).
Here’s a simple way to think about it:
Ionic compounds: Formed by the transfer of electrons from a metal to a non-metal.
Covalent compounds: Formed by the sharing of electrons between two non-metals.
Since NH4Cl has a metal (ammonium) and a non-metal (chlorine), it fits the definition of an ionic compound.
Is CaCl2 ionic or covalent?
Calcium chloride is formed by the ionic bond between a calcium atom and two chlorine atoms. This bond forms because of the significant difference in electronegativity between calcium and chlorine. Calcium, being a metal, readily loses two electrons to achieve a stable electron configuration, becoming a positively charged cation (Ca2+). On the other hand, chlorine, a nonmetal, readily gains one electron to achieve a stable electron configuration, becoming a negatively charged anion (Cl-). The strong electrostatic attraction between the positively charged calcium cation and the negatively charged chlorine anions forms the ionic bond in calcium chloride.
Think of it like this: calcium is like a generous friend who loves to share, giving away its electrons. Chlorine, on the other hand, is like someone who always wants more, happily accepting those electrons. This exchange creates a strong bond between them, forming calcium chloride.
This exchange of electrons between the atoms is the key characteristic that defines ionic bonds. The result is a compound with a neutral overall charge, where the positively charged cations and negatively charged anions are held together in a crystal lattice structure.
To summarize:calcium chloride (CaCl2) is ionic due to the electrostatic attraction between the positively charged calcium cation (Ca2+) and the negatively charged chlorine anions (Cl-), forming a stable crystal lattice.
Is NH4Cl Electrovalent or covalent?
You’re right, NH4Cl has a fascinating combination of covalent, coordinate, and electrovalent bonds.
Let’s delve into the details:
Covalent Bonds: These bonds occur when atoms share electrons to achieve stability. In NH4Cl, the nitrogen atom forms covalent bonds with four hydrogen atoms. This creates the ammonium ion (NH4+).
Coordinate Bonds: In a coordinate bond, both electrons in the bond are donated by the same atom. In NH4Cl, the nitrogen atom in the ammonium ion donates its lone pair of electrons to form a coordinate bond with the hydrogen atom.
Electrovalent Bonds: These bonds form due to the electrostatic attraction between oppositely charged ions. The ammonium ion (NH4+) has a positive charge, while the chloride ion (Cl-) has a negative charge. These opposite charges attract each other, forming an electrovalent bond, also known as an ionic bond.
To sum it up, NH4Cl is a compound with a combination of bonds:
Covalent bonds between nitrogen and hydrogen in the ammonium ion.
Coordinate bonds between nitrogen and hydrogen in the ammonium ion.
Electrovalent bonds between the ammonium ion (NH4+) and the chloride ion (Cl-).
This combination of bond types is what makes NH4Cl a fascinating and unique compound!
Is NH4OH ionic or covalent?
The ammonium ion (NH4+) has a positive charge because of the covalent bonds between the nitrogen and hydrogen atoms. The nitrogen atom shares electrons with four hydrogen atoms, but it still has a lone pair of electrons, giving it a positive charge. On the other hand, the hydroxide ion (OH-) has a negative charge due to the shared electrons between the oxygen and hydrogen atoms. This uneven distribution of electrons results in the hydroxide ion having a negative charge.
Now, you might wonder how these two oppositely charged ions interact. The ionic bond forms due to the electrostatic attraction between the positively charged ammonium ion and the negatively charged hydroxide ion.
So, ammonium hydroxide is an example of a compound with both covalent and ionic bonds. The covalent bonds hold the atoms within the ions together, while the ionic bond holds the ions themselves together.
To visualize it, imagine a group of friends hanging out (the atoms in the ions). They’re connected by covalent bonds, which are like strong friendships. Then, a few of these groups (the ions) are drawn together by a mutual attraction (the ionic bond), forming a larger group.
Is AlCl3 ionic or covalent?
Let’s break it down. AlCl3 is formed by the bonding of aluminum (Al) and chlorine (Cl). Aluminum has a +3 charge, and chlorine has a -1 charge. This difference in charge leads to an attraction between the two elements, and they bond together.
Now, here’s where it gets interesting. The bond between aluminum and chlorine isn’t perfectly ionic. Ionic bonds typically form between a metal and a nonmetal where electrons are completely transferred. In covalent bonds, electrons are shared between two nonmetals. In the case of AlCl3, the electronegativity difference between aluminum and chlorine is large enough for the bond to be considered polar covalent. This means that the electrons are not shared equally between the two elements, and the bond has a slight ionic character.
Think of it like this: AlCl3 is like a hybrid car – it gets its power from both gasoline and electricity. Similarly, AlCl3 gets its bonding properties from both ionic and covalent interactions.
In contrast, AlF3 is considered more ionic because fluorine is even more electronegative than chlorine. This larger electronegativity difference results in a more complete transfer of electrons, making the bond more ionic.
Is FeCl3 ionic or covalent?
Ferric chloride (FeCl3) is indeed an ionic compound. This is because it’s formed by the electrostatic attraction between a metal cation (Fe³⁺) and a non-metal anion (Cl⁻).
Here’s why:
Metal and Non-metal: Iron (Fe) is a metal, and chlorine (Cl) is a non-metal. Ionic compounds typically form when metals bond with non-metals.
Electrostatic Attraction: Metals tend to lose electrons, forming positively charged ions (cations), while non-metals gain electrons, forming negatively charged ions (anions). The strong electrostatic attraction between these oppositely charged ions holds the compound together.
Electronegativity Difference: The electronegativity difference between iron and chlorine is large enough to favor the transfer of electrons, leading to the formation of ions.
Ionic bonds are different from covalent bonds, where atoms share electrons to form a molecule.
Let’s delve deeper into the formation of FeCl3:
Iron’s Role: Iron, being a transition metal, can have multiple oxidation states. In FeCl3, iron exists in its +3 oxidation state, meaning it loses three electrons to become the Fe³⁺ cation.
Chlorine’s Role: Chlorine is a highly electronegative element, meaning it strongly attracts electrons. Each chlorine atom gains one electron to become the Cl⁻ anion.
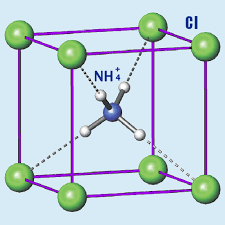
These oppositely charged ions arrange themselves in a crystal lattice structure, where each Fe³⁺ ion is surrounded by six Cl⁻ ions, and each Cl⁻ ion is surrounded by two Fe³⁺ ions. This arrangement maximizes electrostatic attraction and stabilizes the ionic compound.
Understanding the concept of electronegativity and the tendency of metals and non-metals to form ions is crucial in determining whether a compound is ionic or covalent.
Is NaHCO3 ionic or covalent?
Ionic compounds form when a metal like sodium (Na) transfers an electron to a nonmetal, like the bicarbonate ion (HCO3–). This transfer creates oppositely charged ions—a positively charged sodium ion (Na+) and a negatively charged bicarbonate ion (HCO3–). These oppositely charged ions then attract each other, forming a strong ionic bond.
Think of it like magnets—opposite poles attract. In the case of sodium bicarbonate, the positive sodium ions and the negative bicarbonate ions are drawn together in a crystal lattice structure. This structure is held together by the electrostatic forces of attraction between the ions.
In contrast, covalent compounds form when atoms share electrons. This sharing of electrons results in a covalent bond. This is different from the transfer of electrons that occurs in ionic bonds. Since sodium bicarbonate involves the transfer of an electron from sodium to the bicarbonate ion, it falls under the category of ionic compounds.
So, the next time you use baking soda for your baking needs, remember that it’s a fascinating example of an ionic compound in action!
Is NH4Cl ionic or not?
Ammonium (NH4+) is a positively charged ion, while chloride (Cl-) is negatively charged. These ions form a bond through electrostatic attraction, which is a characteristic of ionic compounds. You can think of it like magnets—opposites attract!
However, NH4+ has already formed four covalent bonds with the hydrogen atoms, making it a stable unit. This means there are no more electrons available for direct covalent bonding with Cl-. Instead, the attraction between the NH4+ cation and the Cl- anion forms the ionic bond.
Here’s a more detailed explanation of why NH4Cl is ionic:
Formation of ammonium ion (NH4+): The nitrogen atom in ammonia (NH3) has a lone pair of electrons. When it reacts with a proton (H+), a coordinate covalent bond is formed, where the nitrogen atom contributes both electrons to the bond. This forms the ammonium ion (NH4+).
Electrostatic attraction: The positively charged ammonium ion is attracted to the negatively charged chloride ion, forming an ionic bond.
Ionic lattice structure: The ammonium chloride compound doesn’t exist as individual molecules but rather as a three-dimensional lattice structure where each ammonium ion is surrounded by chloride ions, and vice versa.
This arrangement results in a compound with distinct properties that are typical of ionic compounds, such as high melting point, good conductivity in solution, and a crystal structure.
Is NH4 ionic or covalent?
Think of it like this: the bonds between the nitrogen atom and the four hydrogen atoms within the NH4+ molecule are covalent. These bonds are formed by the sharing of electrons between the atoms. However, the NH4+ molecule as a whole has lost an electron, resulting in a positive charge. This positive charge allows it to attract negatively charged ions, forming ionic bonds with them.
A good way to visualize this is to think of the NH4+ molecule as a single entity with a positive charge. This charged entity then interacts with other ions through electrostatic forces, forming ionic bonds. It’s important to remember that the nature of the bond depends on the overall charge of the molecule or ion, not just the individual bonds within it.
Let me give you a concrete example: Imagine a single NH4+ ion. It’s positively charged, right? Now, imagine a Cl- ion, which is negatively charged. When these two ions come together, the positive charge of NH4+ will attract the negative charge of Cl-, forming an ionic bond. The covalent bonds within the NH4+ molecule stay intact, while the ionic bond forms between the NH4+ molecule and the Cl- ion.
It’s like two people holding hands (covalent bonds within the NH4+ molecule) and then one of them holding a third person’s hand (the ionic bond formed between NH4+ and Cl-).
See more here: Is Nh4Cn Ionic Or Covalent? | Is Nh4Cl An Ionic Compound
Is NH4Cl an ionic compound?
You’re right, ammonium chloride is indeed ionic. But the way it forms is a bit different from a typical metal-nonmetal bond. Here’s why:
Ammonium chloride forms when ammonia (NH3) reacts with hydrogen chloride (HCl). The ammonia molecule grabs a hydrogen ion (H+) from the hydrogen chloride, creating a positively charged ammonium ion (NH4+) and a negatively charged chloride ion (Cl-). These oppositely charged ions are then attracted to each other, forming the ionic compound ammonium chloride (NH4Cl).
So, although we don’t have a classic metal-nonmetal bond, the ammonium ion and chloride ion still have a strong electrostatic attraction, which is the hallmark of ionic compounds.
Let’s break this down further:
Ammonia (NH3) is a neutral molecule. It has a lone pair of electrons on the nitrogen atom.
Hydrogen chloride (HCl) is a covalent compound, but it readily dissociates in solution to form hydrogen ions (H+) and chloride ions (Cl-).
* The lone pair on the nitrogen atom in ammonia attracts the hydrogen ion from HCl.
* This forms a coordinate covalent bond between the nitrogen atom in ammonia and the hydrogen ion.
* The resulting molecule is ammonium ion (NH4+), which carries a +1 charge.
* The chloride ion (Cl-) from the HCl molecule balances the positive charge of the ammonium ion, resulting in the formation of ammonium chloride (NH4Cl).
The key takeaway here is that although we don’t have a traditional metal-nonmetal bond in ammonium chloride, the strong electrostatic attraction between the positively charged ammonium ion and the negatively charged chloride ion makes it a true ionic compound.
What is NH4Cl?
Ammonium chloride, also known as sal ammoniac, is a common inorganic compound with the chemical formula NH4Cl. It’s formed from the reaction of ammonia (NH3) and hydrochloric acid (HCl). While it’s a by-product of sodium carbonate production, ammonium chloride has many other uses.
Think of it as a versatile ingredient in various industries. Ammonium chloride is used in fertilizers to provide nitrogen, an essential plant nutrient. It also plays a role in dry-cell batteries, acting as an electrolyte. In the metal industry, ammonium chloride aids in cleaning metal surfaces, and you’ll find it in some food additives, too!
Its applications are diverse, and it’s a fascinating example of how a simple chemical compound can have a big impact on various fields.
Is NH4 ion ionic or covalent?
You’re right to wonder if the bonds within NH4+ are ionic or covalent. The answer is: it’s a bit of both.
Here’s the breakdown:
The bonds between nitrogen and hydrogen atoms are covalent. This means the nitrogen and hydrogen atoms share electrons to form a stable bond. They’re like two friends sharing a pizza, each getting a slice.
The NH4+ ion as a whole is ionic. It carries a positive charge because it has lost one electron. This is why NH4+ ion can form ionic bonds with negatively charged ions. Think of NH4+ ion as a hungry friend who needs a slice of pizza (an electron) to be happy.
Let’s take a closer look at how the NH4+ ion forms:
Imagine a humble ammonia molecule (NH3) with a nitrogen atom bonded to three hydrogen atoms. Nitrogen has five valence electrons, and it shares one with each hydrogen atom to complete its octet (eight electrons in the outer shell). That’s where the covalent part comes in.
Now, this NH3 molecule can actually gain another hydrogen ion (H+). This is where it gets interesting! When a H+ ion approaches, it attracts the lone pair of electrons on the nitrogen atom. This lone pair of electrons is essentially donated to the H+ ion, forming a fourth bond. The nitrogen atom now has four bonds, making it a positively charged NH4+ ion. This is where the ionic part comes in.
In essence, the NH4+ ion is a bit of a hybrid! It has strong covalent bonds between the nitrogen and hydrogen atoms, but it also has an overall positive charge due to the donation of an electron. This positive charge allows it to form ionic bonds with other negatively charged species like chlorine ion (Cl-) to form compounds like ammonium chloride (NH4Cl).
So, there you have it! NH4+ ion is a complex but fascinating example of how covalent and ionic bonding work together in a molecule. It’s a great example of how chemistry can be both intricate and beautiful!
Is NH4Cl a halogen?
NH4Cl is made up of NH4+ and Cl-. NH4+ is called ammonium and Cl- is called chloride. When we combine these two ions, we get ammonium chloride.
Halogens are a group of elements that include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are found in Group 17 of the periodic table. They are highly reactive and readily form negative ions, also known as halides, by gaining one electron.
While Cl is a halogen, NH4Cl is not. NH4Cl is an ionic compound that’s formed by the electrostatic attraction between the NH4+ cation and the Cl- anion.
Think of it this way: NH4Cl is like a salt, where the Cl- is the “salt” part, and the NH4+ is the “flavor” that makes it unique. Cl- is the halide ion that makes the compound a salt.
NH4Cl is a white crystalline solid that’s commonly used as a fertilizer, a food additive, and a component in some types of batteries. It’s important to note that NH4Cl is not a halogen itself, but it does contain a halogen element, chlorine, in the form of a chloride ion.
See more new information: bmxracingthailand.com
Is Nh4Cl An Ionic Compound: Understanding The Bonds
First off, let’s get the basics straight. Ionic compounds are formed when a metal and a nonmetal interact, resulting in the transfer of electrons. This transfer creates ions, which are charged particles. The metal loses electrons and becomes a positively charged cation, while the nonmetal gains electrons and becomes a negatively charged anion. These opposite charges attract each other, forming a strong electrostatic bond.
Now, let’s take a closer look at NH4Cl. It’s made up of ammonium (NH4+) and chloride (Cl-) ions. The ammonium ion is a polyatomic cation, meaning it’s made up of multiple atoms, in this case, nitrogen and hydrogen, bonded together. The chloride ion is a monatomic anion, consisting of just one chlorine atom.
You might be wondering if ammonium is a metal. Well, it’s not! Ammonium is a polyatomic ion, which means it acts like a metal in this situation, forming a cation.
So, is NH4Cl an ionic compound? Yes! It is! We have a cation (NH4+) and an anion (Cl-), held together by the strong electrostatic force between them.
Understanding the Nature of Bonds in NH4Cl
Let’s break down why NH4Cl is considered an ionic compound even though ammonium is a polyatomic ion:
1. The Formation of Ammonium Ion: The nitrogen atom in ammonium shares electrons with four hydrogen atoms. This sharing creates covalent bonds, but the nitrogen atom ends up with a positive charge as it doesn’t have an equal share of electrons.
2. The Interaction of Ammonium and Chloride Ions: The positively charged ammonium ion is attracted to the negatively charged chloride ion. This strong electrostatic attraction is the defining feature of an ionic bond.
3. The Role of Electrostatic Attraction: The force holding the ammonium and chloride ions together is a strong electrostatic force. This force is what gives ionic compounds their characteristic properties, such as high melting and boiling points.
Key Characteristics of NH4Cl as an Ionic Compound:
Here are some important properties of NH4Cl that confirm its ionic nature:
– High Melting and Boiling Points:Ionic compounds like NH4Cl have high melting and boiling points because the electrostatic forces holding them together are very strong.
– Solubility in Water: NH4Cl dissolves readily in water. This is because the water molecules can surround the ions, weakening the electrostatic attraction between them and allowing them to break apart.
– Electrical Conductivity in the Molten or Dissolved State:Ionic compounds conduct electricity when molten or dissolved in water because the ions are free to move and carry the electric charge.
Why Does This Matter?
Understanding whether a compound is ionic or covalent is crucial in chemistry. It helps us predict how a compound will behave:
Predicting Chemical Reactions: The type of bond (ionic or covalent) influences how a compound reacts with other substances.
Understanding Physical Properties: The type of bond determines many physical properties, such as melting point, boiling point, and solubility.
FAQs:
Here are some frequently asked questions about NH4Cl:
1. What is the Chemical Formula of Ammonium Chloride?
The chemical formula for ammonium chloride is NH4Cl.
2. Is Ammonium Chloride a Strong or Weak Electrolyte?
Ammonium chloride is a strong electrolyte, meaning it dissociates completely into ions when dissolved in water.
3. What are the Uses of Ammonium Chloride?
Ammonium chloride has various applications:
Fertilizer: Ammonium chloride is a source of nitrogen, an essential nutrient for plant growth.
Food Additive: It’s used as a yeast nutrient in baking.
Medicine: It’s used in cough syrups and as an expectorant.
Metallurgy: It’s used in the extraction and refining of metals.
4. Is Ammonium Chloride a Solid, Liquid, or Gas at Room Temperature?
Ammonium chloride is a white crystalline solid at room temperature.
5. Is Ammonium Chloride Acidic, Basic, or Neutral?
Ammonium chloride is slightly acidic. This is because when it dissolves in water, it forms ammonium ions, which can donate a proton (H+) to water, increasing the acidity.
6. Is Ammonium Chloride Toxic?
Ammonium chloride can be toxic in large amounts. It’s important to handle it carefully and keep it away from children and pets.
7. Is Ammonium Chloride Flammable?
Ammonium chloride is not flammable. It’s a solid and doesn’t burn.
8. How is Ammonium Chloride Made?
Ammonium chloride is typically produced by reacting ammonia with hydrochloric acid. This reaction results in the formation of ammonium ions and chloride ions, which combine to form ammonium chloride.
9. Where Can I Find Ammonium Chloride?
You can find ammonium chloride at many retailers, including garden centers, hardware stores, and online retailers.
I hope this detailed explanation helps you understand why NH4Cl is an ionic compound and gives you a better grasp of its properties and applications.
Is NH4Cl (Ammonium chloride) Ionic or Covalent? – YouTube
To tell if NH4Cl (Ammonium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Na is a metal and Cl is a group of non-metals. Here the group… YouTube
electronegativity – Why does ammonium chloride form
Why does $\ce{NH4Cl}$ form an ionic bond if the difference in electronegativity between nitrogen and chlorine is $0$? Shouldn’t it be at least 1.7 in order for an ionic bond to form? If not what is the reason Chemistry Stack Exchange
NH4Cl Bond Types (Ionic, Covalent, and Coordinate Covalent)
The overall compound is considered ionic because of the NH4+ and Cl- ions being electrostatically attracted. Within the NH4+ ion there are covalent bonds, one of YouTube
ionic compounds – How is NH4Cl a salt? – Chemistry Stack
So what I know is, a salt is an ionic compound between a metal and a non-metal which exchange electrons. Like in $\ce{NaCl}$, $\ce{Na}$ is the metal and Chemistry Stack Exchange
3.5: Ionic Compounds- Formulas and Names – Chemistry
Ionic Compounds. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the Chemistry LibreTexts
What is the ionic compound name for nh4cl? | Socratic
NH4Cl is an ionic compound made up of ammonium (NH4+) and chloride (Cl-) ions. The name of the compound is derived from the suffixes of the ions: -ine for Socratic
Ammonium Chloride (NH4Cl) – Structure, Properties,
Ammonium chloride is an inorganic compound with the chemical name Ammonium Chloride. It is also known as sal ammoniac, the salt of ammonia and hydrogen chloride. BYJU’S
4.2 Ionic and Covalent Compounds – Chemistry LibreTexts
A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are Chemistry LibreTexts
Is Nh4Cl (Ammonium Chloride) Ionic Or Covalent?
Nh4Cl Bond Types (Ionic, Covalent, And Coordinate Covalent)
Lewis Structure Of Nh4Cl (Ammonium Chloride, Ionic)
How To Write The Name For Nh4Cl
The Number Of Ionic, Convalent And Coordinate Bonds In `Nh_(4)Ci` Are Respectively
Ionic And Covalent Bonds | Chemical Bonding
Gcse Chemistry – What Is Ionic Bonding? How Does Ionic Bonding Work? Ionic Bonds Explained #14
Naming Ionic Compounds
Lewis Structure Of Nh4Cl, Ammonium Chloride
Gcse Chemistry – What Is An Ionic Compound? Ionic Compounds Explained #15
Link to this article: is nh4cl an ionic compound.
See more articles in the same category here: bmxracingthailand.com/what
