What intermolecular forces are in 1 Pentanol?
1-pentanol is a fascinating molecule. It’s an alcohol, meaning it has a hydroxyl group (-OH) attached to its carbon chain. This hydroxyl group plays a starring role in determining its intermolecular forces.
You’re right, hydrogen bonding is definitely a key player in 1-pentanol. The hydroxyl group’s hydrogen atom forms strong hydrogen bonds with the oxygen atom of a neighboring 1-pentanol molecule. This is like a strong magnet attracting other molecules, making 1-pentanol have a higher boiling point compared to similar hydrocarbons without the hydroxyl group.
But it’s not just about hydrogen bonding. 1-pentanol also experiences dipole-dipole interactions and London dispersion forces.
Dipole-dipole interactions occur because of the uneven distribution of electrons within the molecule, leading to a slight positive charge on one end and a slight negative charge on the other. These opposite charges attract each other, like miniature magnets.
London dispersion forces are the weakest type of intermolecular force but they are present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles that attract each other.
So, 1-pentanol experiences a trio of intermolecular forces: hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The hydrogen bonding, with its strong attractive force, plays the most significant role in determining 1-pentanol’s properties. This strong attraction explains why 1-pentanol has a relatively high boiling point and is more soluble in water compared to hydrocarbons without the hydroxyl group.
What are the intermolecular forces between water and Pentanol?
London dispersion forces, dipole-dipole interactions, and hydrogen bonding are all present between water and pentanol.
Hydrogen bonding is the strongest of these forces. It occurs when a hydrogen atom is bonded to a highly electronegative atom, like oxygen. In water, the hydrogen atoms are attracted to the oxygen atoms in pentanol.
Dipole-dipole interactions are weaker than hydrogen bonds. They occur between polar molecules, like water and pentanol. The positive end of one molecule is attracted to the negative end of another molecule.
London dispersion forces are the weakest of the three. They occur between all molecules, even non-polar molecules. They are caused by temporary fluctuations in electron density.
The presence of these intermolecular forces explains why pentanol is slightly soluble in water. The hydrogen bonding between water and pentanol molecules helps to overcome the London dispersion forces and dipole-dipole interactions that would otherwise keep the two molecules separate.
However, pentanol is not completely soluble in water. This is because the pentanol molecule has a long, non-polar hydrocarbon chain. This chain is not attracted to the polar water molecules, and it makes it difficult for pentanol to dissolve in water.
In summary, pentanol and water are able to interact due to a combination of intermolecular forces, including hydrogen bonding, dipole-dipole interactions, and London dispersion forces. While hydrogen bonding between water and pentanol is strong, the long, non-polar hydrocarbon chain of pentanol limits its overall solubility in water.
What intermolecular forces are present in liquid pentanol?
Dispersion forces, hydrogen bonding, and dipole-dipole forces are all present in liquid pentanol.
Dispersion forces are the weakest type of intermolecular force and are present in all molecules. These forces arise from temporary fluctuations in electron distribution around a molecule, creating temporary dipoles that induce dipoles in neighboring molecules. Pentanol, with its relatively large size and nonpolar hydrocarbon chain, experiences significant dispersion forces.
Dipole-dipole forces occur between polar molecules, where permanent dipoles attract each other. While pentanol’s hydrocarbon chain is nonpolar, the presence of the hydroxyl group (OH) makes the molecule polar. The oxygen atom in the hydroxyl group is more electronegative than the hydrogen atom, resulting in a partial negative charge on the oxygen and a partial positive charge on the hydrogen. These partial charges create a dipole moment in the pentanol molecule.
Hydrogen bonding, the strongest type of dipole-dipole interaction, is present in pentanol due to the presence of the hydroxyl group. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom, like oxygen, and interacts with a lone pair of electrons on another oxygen atom in a neighboring molecule. In pentanol, the hydrogen atom in the hydroxyl group forms a hydrogen bond with the oxygen atom of another pentanol molecule.
The presence of these intermolecular forces significantly impacts the physical properties of pentanol. The strong hydrogen bonding in pentanol leads to a relatively high boiling point compared to similar-sized hydrocarbons. Additionally, the polarity of the molecule contributes to its solubility in polar solvents like water.
What is the strongest intermolecular force in 1 propanol?
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. These bonds are stronger than typical dipole-dipole interactions because the electronegativity difference between the hydrogen atom and the electronegative atom creates a very strong partial positive charge on the hydrogen atom. This strong partial positive charge is then strongly attracted to the lone pairs of electrons on the electronegative atom of another molecule.
In 1-propanol, the hydrogen bonding is so strong that it significantly affects the physical properties of the molecule. For example, 1-propanol has a higher boiling point than other similar molecules, such as propane, because the hydrogen bonds require more energy to break. 1-propanol is also more soluble in water than other similar molecules because the hydrogen bonds can form between the 1-propanol molecules and the water molecules.
Let’s delve a little deeper into why hydrogen bonding is so significant. The strength of an intermolecular force directly influences a molecule’s physical properties, like melting point, boiling point, and solubility.
Here’s a breakdown:
Higher Boiling Point: Because hydrogen bonds are strong, a lot of energy is needed to overcome them and break the intermolecular attractions. This means 1-propanol needs a higher temperature (boiling point) to transition from a liquid to a gas.
Increased Solubility: The strong attraction between the partially positive hydrogen of 1-propanol and the partially negative oxygen of water allows the two molecules to readily mix. This means 1-propanol will be more soluble in water than in a non-polar solvent where hydrogen bonding isn’t possible.
It’s important to remember that hydrogen bonding is just one of many intermolecular forces. However, it is typically the strongest one present. Understanding hydrogen bonding helps us explain and predict the behavior of many substances, making it a crucial concept in chemistry.
What are the molecules in 1-pentanol?
1-Pentanol, also known as n-pentanol or pentan-1-ol, is an organic compound with the chemical formula CH3CH2CH2CH2CH2OH. It’s classified as a primary alcohol, which means the hydroxyl group (OH) is attached to a carbon atom that’s only bonded to one other carbon atom.
1-Pentanol is a colorless liquid with a distinctive aroma. It’s one of eight isomers with the formula C5H11OH. Isomers are molecules that have the same chemical formula but different arrangements of atoms.
Let’s dive deeper into the molecules that make up 1-pentanol:
Carbon (C): The backbone of 1-pentanol is a chain of five carbon atoms. Each carbon atom forms four bonds, allowing it to connect to other carbon atoms and hydrogen atoms.
Hydrogen (H): Hydrogen atoms are attached to each carbon atom in the chain. They’re the most abundant element in 1-pentanol.
Oxygen (O): The hydroxyl group (OH) contains one oxygen atom. This group is what gives 1-pentanol its characteristic properties as an alcohol.
Here’s a simplified way to visualize the arrangement of atoms in 1-pentanol:
Carbon chain: Imagine a straight chain of five beads, representing the carbon atoms.
Hydrogen atoms: Each bead (carbon) has two or three smaller beads (hydrogen) attached to it.
Hydroxyl group: At one end of the carbon chain, a bead representing oxygen is attached to a single hydrogen atom. This is the hydroxyl group (OH).
Understanding these individual molecules and how they’re connected helps us understand the properties and reactions of 1-pentanol.
What are the intermolecular forces in 1 pentane?
London Dispersion Forces are temporary, weak attractions that arise from the constant motion of electrons in molecules. As electrons move, they create temporary, fluctuating dipoles, which induce similar dipoles in neighboring molecules. These temporary dipoles then attract each other, resulting in a weak intermolecular force.
You might be wondering why other intermolecular forces, like dipole-dipole interactions or hydrogen bonding, are not present in pentane. Here’s why:
Dipole-dipole interactions occur between polar molecules, meaning molecules with a permanent separation of charge due to an uneven distribution of electrons. Pentane, however, is a nonpolar molecule, with a symmetrical structure and an even distribution of electrons. This lack of polarity prevents dipole-dipole interactions from forming.
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, like oxygen, nitrogen, or fluorine. Pentane doesn’t have any hydrogen atoms directly bonded to these electronegative atoms, so hydrogen bonding isn’t possible.
In summary, because pentane is a nonpolar molecule without any hydrogen atoms bonded to highly electronegative atoms, it only experiences London Dispersion Forces as intermolecular interactions. These weak forces are the primary factor influencing the physical properties of pentane, such as its boiling point and melting point.
What intermolecular forces are in 1-butanol?
Hydrogen bonding, dipole-dipole forces, and London dispersion forces are all present in 1-butanol.
Hydrogen bonding is the strongest of these forces. It occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, and is attracted to an electron pair on a neighboring oxygen atom. This strong attraction is what makes 1-butanol a liquid at room temperature.
Dipole-dipole forces arise from the uneven distribution of electrons within a molecule. In 1-butanol, the oxygen atom is more electronegative than the carbon and hydrogen atoms, creating a partial negative charge on the oxygen atom and a partial positive charge on the carbon and hydrogen atoms. These opposite charges attract each other, leading to dipole-dipole interactions.
Finally, London dispersion forces are the weakest type of intermolecular force. They occur due to temporary fluctuations in electron distribution around an atom or molecule, creating temporary dipoles. These temporary dipoles induce dipoles in neighboring molecules, resulting in weak attractions.
These three types of intermolecular forces work together to influence the physical properties of 1-butanol, such as its boiling point, melting point, and solubility.
Here’s a bit more about these forces:
Hydrogen bonding: This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is directly bonded to a highly electronegative atom, like oxygen, nitrogen, or fluorine. These interactions are particularly strong because the small size of the hydrogen atom allows for close proximity between the interacting atoms.
Dipole-dipole forces: These forces arise from the permanent dipoles within polar molecules. Polar molecules have an uneven distribution of electrons, resulting in a partial positive charge at one end of the molecule and a partial negative charge at the other. These opposite charges attract each other, leading to dipole-dipole interactions.
London dispersion forces: These forces are present in all molecules, regardless of whether they are polar or nonpolar. They arise from temporary fluctuations in electron distribution around an atom or molecule, creating temporary dipoles. These temporary dipoles induce dipoles in neighboring molecules, resulting in weak attractions. The strength of London dispersion forces increases with the size of the molecule and the number of electrons.
Understanding these intermolecular forces helps us to predict the behavior of 1-butanol and other molecules in a variety of situations.
What interparticle forces are in 2 Pentanol?
2-pentanol exhibits hydrogen bonding, a strong intermolecular force that arises from the interaction between a hydrogen atom covalently linked to a highly electronegative atom like oxygen, and an electron pair in the adjacent molecule. Because of the presence of the hydroxyl group (-OH), 2-pentanol can participate in hydrogen bonding, significantly impacting its properties, such as its boiling point and solubility.
2-pentanol also exhibits London dispersion forces, a type of van der Waals force that occurs due to temporary fluctuations in electron distribution around molecules. These forces are present in all molecules, but they are weaker than hydrogen bonding.
However, it’s important to note that 2-pentanol is not a polar molecule. This is because the non-polar alkyl chain in 2-pentanol counteracts the polar hydroxyl group. As a result, 2-pentanol does not exhibit dipole-dipole interactions, which are intermolecular forces that occur between polar molecules.
Now, let’s explore why hydrogen bonding is such a significant factor for 2-pentanol. Hydrogen bonds are stronger than other intermolecular forces because they involve a direct interaction between a hydrogen atom and an electron pair, resulting in a strong electrostatic attraction. This strength is reflected in 2-pentanol’s relatively high boiling point compared to other molecules of similar size without hydrogen bonding.
Understanding the interparticle forces in 2-pentanol helps us predict its behavior and properties. For instance, hydrogen bonding accounts for its higher boiling point and its ability to dissolve in polar solvents like water. Keep in mind that the presence of hydrogen bonding, in addition to other intermolecular forces, plays a crucial role in shaping the physical and chemical characteristics of 2-pentanol.
See more here: What Are The Intermolecular Forces Between Water And Pentanol? | Select All The Intermolecular Forces Associated With 1-Pentanol.
What are the three types of intermolecular forces?
We’ll focus on three main types of intermolecular forces: dispersion forces, dipole-dipole forces, and hydrogen bonds.
Dispersion forces (also called London forces) are the weakest type of intermolecular force. They arise from temporary fluctuations in electron distribution within a molecule. Imagine a molecule with its electrons evenly distributed. At a given moment, the electrons might shift slightly, creating a temporary instantaneous dipole. This temporary dipole can then induce a temporary dipole in a neighboring molecule. These fleeting attractions are called dispersion forces. Think of it like a fleeting moment of attraction between two people who accidentally bump into each other. They might share a quick smile and then move on, but that brief connection was still there.
Let’s break down how dispersion forces work:
Temporary Dipole: Imagine a molecule like a tiny, spinning top. As the top spins, its electrons move around, creating a momentary imbalance in the electron distribution. This creates a temporary dipole with a slightly positive end and a slightly negative end.
Induced Dipole: Now, let’s bring in a second molecule. The temporary dipole in the first molecule can influence the electron distribution in the second molecule. This leads to the formation of an induced dipole in the second molecule.
Attraction: The induced dipole in the second molecule is now attracted to the instantaneous dipole in the first molecule, leading to a weak attraction between them.
Dispersion forces are present in all molecules, but they become stronger with increasing molecular size and surface area. This is because larger molecules have more electrons that can participate in these temporary interactions. Think of it this way: a larger molecule is like a bigger spinning top with more electrons swirling around. The more electrons you have, the more opportunities there are for temporary dipoles and induced dipoles to form.
How are physical properties determined by intermolecular forces?
We’ll focus on three main types of intermolecular forces:
Dispersion forces: These are the weakest forces, present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles.
Dipole-dipole forces: These occur between polar molecules, which have permanent dipoles. The positive end of one molecule is attracted to the negative end of another.
Hydrogen bonds: These are the strongest type of intermolecular force, occurring when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) in one molecule and is attracted to an electron pair on a nearby atom.
Let’s delve a bit deeper into how these forces influence physical properties.
Dispersion forces are responsible for the physical states of many substances. For instance, the strength of dispersion forces increases with molecular size and the number of electrons. Larger molecules have more electrons, which are more easily distorted, leading to stronger temporary dipoles and stronger dispersion forces. This is why larger molecules tend to have higher melting points and boiling points than smaller molecules.
Dipole-dipole forces play a crucial role in the properties of liquids and solids. They lead to higher melting and boiling points compared to substances with only dispersion forces. This is because the stronger attractions between polar molecules require more energy to overcome.
Hydrogen bonds have a significant impact on many properties. For example, water has a relatively high melting point and boiling point due to the strong hydrogen bonds between its molecules. Hydrogen bonding is also responsible for the structure of DNA and proteins, essential components of life.
Remember, these intermolecular forces are constantly working together to influence the physical properties of substances. Understanding them is essential to predicting and explaining how matter behaves!
Why are intermolecular forces not intra molecular forces?
Intramolecular forces are the forces that hold atoms together within a molecule. These are the strong chemical bonds, like covalent or ionic bonds, that make up the molecule’s structure. Think of it like the glue that holds a house together. Without these bonds, the molecule wouldn’t exist!
Intermolecular forces, on the other hand, are the forces of attraction *between* molecules. These are weaker than intramolecular forces and determine how molecules interact with each other. Imagine these forces as the ties that hold houses in a neighborhood together. They’re not as strong as the glue inside each house, but they’re still important for keeping the whole community organized.
Here are a few examples of how intermolecular forces affect the properties of a substance:
Boiling point: Substances with strong intermolecular forces have higher boiling points because more energy is needed to overcome the attraction between molecules and allow them to escape into the gas phase. Water, for instance, has strong hydrogen bonds, which is why it has a relatively high boiling point compared to other molecules.
Solubility: Substances with similar intermolecular forces tend to dissolve in each other. For example, water is a polar molecule with strong hydrogen bonds and it readily dissolves other polar molecules like sugar. However, it doesn’t mix well with nonpolar molecules like oil, which have weak intermolecular forces.
Viscosity: Liquids with strong intermolecular forces are more viscous (thicker). Imagine honey, which has strong hydrogen bonds, flowing more slowly than water.
In essence, while intramolecular forces define the *internal* structure of a molecule, intermolecular forces govern the *interactions* between molecules, which play a big role in how matter behaves. So, the next time you’re sipping a cup of coffee or marveling at the fluidity of honey, remember the subtle but powerful forces at play!
Why is intermolecular force important in organic chemistry?
Boiling Point
Think of boiling point as the temperature where a liquid turns into a gas. Strong intermolecular forces mean that it takes more energy to separate the molecules, so the boiling point is higher. Weak intermolecular forces mean that it takes less energy to separate the molecules, so the boiling point is lower.
For example, let’s compare water and methane. Water has strong hydrogen bonds which is a type of intermolecular force. Methane has weaker Van der Waals forces. Water has a boiling point of 100 degrees Celsius, while methane has a boiling point of -161.5 degrees Celsius. This big difference in boiling point is due to the strength of the intermolecular forces between the molecules.
Solubility
Solubility is all about how well one substance dissolves in another. When a substance dissolves, the molecules of the solute (the substance that dissolves) are surrounded by molecules of the solvent (the substance that does the dissolving). This happens because the intermolecular forces between the solute and solvent molecules are stronger than the intermolecular forces between the solute molecules themselves.
For example, water is a polar molecule and has strong hydrogen bonds. Salt (NaCl) is also a polar molecule and has strong ionic bonds. When salt dissolves in water, the hydrogen bonds between the water molecules and the ionic bonds between the sodium and chloride ions form strong attractions, resulting in the salt dissolving. However, oil is a nonpolar molecule and has weak Van der Waals forces. When oil is mixed with water, the hydrogen bonds between the water molecules are stronger than the Van der Waals forces between the oil and water molecules. This means the oil and water molecules don’t mix well and the oil doesn’t dissolve in water.
So, understanding intermolecular forces helps us predict how substances will behave, which is super helpful in organic chemistry!
See more new information: bmxracingthailand.com
Select All Intermolecular Forces In 1-Pentanol
1-pentanol, also known as n-pentanol, is an alcohol with the chemical formula CH3(CH2)4OH. It’s got a hydrocarbon chain attached to a hydroxyl group (-OH), and that’s where the fun begins.
Intermolecular Forces: The Glue Holding Molecules Together
Intermolecular forces are like the invisible glue that holds molecules together. These forces are much weaker than the intramolecular forces that hold atoms together within a molecule, but they still play a huge role in determining a substance’s properties like melting point, boiling point, and solubility.
There are three main types of intermolecular forces we need to consider:
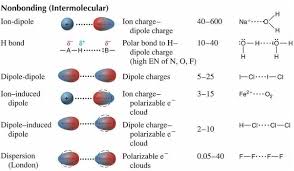
Hydrogen Bonding: This is the strongest type of intermolecular force. It happens when a hydrogen atom is bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine. The hydrogen atom gets a slight positive charge and forms a dipole-dipole interaction with the lone pair of electrons on the other electronegative atom. Think of it like a special kind of attraction between hydrogen and these super-attracting atoms.
Dipole-Dipole Interactions: These forces happen between polar molecules. Remember, polar molecules have a permanent dipole moment, meaning one end of the molecule has a slight positive charge and the other end has a slight negative charge. These charges attract each other, leading to dipole-dipole interactions.
London Dispersion Forces: These are the weakest type of intermolecular force and occur between all molecules, even nonpolar molecules. These forces arise from temporary fluctuations in electron distribution around the molecule. Imagine electrons moving around and creating temporary, fleeting dipoles. These temporary dipoles can then interact with other temporary dipoles on nearby molecules.
Let’s Talk About 1-Pentanol
Now, back to our friend 1-pentanol. It’s got all three types of intermolecular forces going on:
Hydrogen Bonding: The hydroxyl group (-OH) in 1-pentanol is the key player here. This group has a hydrogen atom attached to an oxygen atom, creating a hydrogen bond with another 1-pentanol molecule. Think of it as a chain where each molecule is linked to its neighbor through a hydrogen bond. This strong hydrogen bonding is why 1-pentanol has a relatively high boiling point compared to similar hydrocarbons.
Dipole-Dipole Interactions: The hydroxyl group (-OH) in 1-pentanol makes the molecule polar. This means it has a dipole moment, and 1-pentanol molecules can interact with each other through dipole-dipole interactions.
London Dispersion Forces: These forces are always present, even in nonpolar molecules. In 1-pentanol, these forces contribute to the overall attraction between molecules. Although the London dispersion forces are weaker than the other types of intermolecular forces in 1-pentanol, they still play a role in holding the molecules together.
The Big Picture: Why Do We Care About Intermolecular Forces?
So, why are we so interested in all these intermolecular forces? It’s because they have a huge impact on a molecule’s properties:
Boiling Point: Stronger intermolecular forces mean a higher boiling point. 1-pentanol has a higher boiling point than similar hydrocarbons because of the hydrogen bonding present.
Melting Point: Similar to boiling point, intermolecular forces play a role in melting point. Stronger intermolecular forces tend to lead to a higher melting point.
Solubility: 1-pentanol is polar and can form hydrogen bonds with other polar molecules like water. This is why it’s soluble in water. However, 1-pentanol is not very soluble in nonpolar solvents like hexane because the intermolecular forces between 1-pentanol and hexane are weak.
FAQs About 1-Pentanol and Intermolecular Forces
Q: What is the strongest intermolecular force present in 1-pentanol?
A: The strongest intermolecular force present in 1-pentanol is hydrogen bonding, thanks to the hydroxyl group (-OH) which forms hydrogen bonds with other 1-pentanol molecules.
Q: Why does 1-pentanol have a higher boiling point than pentane?
A:1-pentanol has a higher boiling point than pentane because of hydrogen bonding. Pentane is a nonpolar molecule and only experiences London dispersion forces. Since hydrogen bonding is a much stronger intermolecular force than London dispersion forces, more energy is required to overcome the attractions between 1-pentanol molecules, leading to a higher boiling point.
Q: Is 1-pentanol soluble in water?
A: Yes, 1-pentanol is soluble in water. 1-pentanol is a polar molecule and can form hydrogen bonds with water molecules.
Q: What are some other examples of molecules that exhibit hydrogen bonding?
A: Hydrogen bonding is common in molecules that contain hydrogen bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine. Some examples include water (H2O), ammonia (NH3), and ethanol (CH3CH2OH).
Q: How can I predict the types of intermolecular forces present in a molecule?
A: You can predict the types of intermolecular forces present in a molecule by considering the following:
Polarity: Polar molecules will have dipole-dipole interactions, and if they have a hydrogen atom bonded to an electronegative atom, they will also have hydrogen bonding.
Molecular Shape: Molecular shape can also influence the strength of intermolecular forces. For example, linear molecules tend to have stronger London dispersion forces than spherical molecules.
Electronegativity: Look for hydrogen bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine to identify hydrogen bonding.
Q: Why are intermolecular forces important?
A:Intermolecular forces are super important because they influence a substance’s physical properties, including its:
Boiling Point: Stronger intermolecular forces lead to higher boiling points.
Melting Point: Similar to boiling point, stronger intermolecular forces mean a higher melting point.
Solubility:Intermolecular forces determine a substance’s solubility in different solvents.
Viscosity: Stronger intermolecular forces lead to higher viscosity.
Surface Tension: Stronger intermolecular forces lead to higher surface tension.
So, there you have it. 1-pentanol is a molecule that exhibits all three types of intermolecular forces – hydrogen bonding, dipole-dipole interactions, and London dispersion forces. Understanding these forces is crucial for understanding the physical properties of 1-pentanol and other molecules.
Solved Select all the intermolecular forces associated with – Chegg
Question: Select all the intermolecular forces associated with 1-pentanol? London Disperson Dipole-dipole lon-dipole H-bonding Select all intermolecular forces that Chegg
2.6: Intermolecular Force and Physical … – Chemistry
For organic compounds, hydrogen bonds play important roles in determining the properties of compounds with OH or NH bonds, for example alcohol (R-OH), carboxylic acid (R-COOH), amine (R-NH 2) Chemistry LibreTexts
Select all the intermolecular forces associated wlth 1-pentanol?
We have to choose all intermolecular forces associated with 1-pentanol. a. Hydrogen bonds occur between a very electronegative atom and hydrogen that is attached to another Quizlet
Solved Question 5 3 pts Select all the intermolecular
Science. Chemistry questions and answers. Question 5 3 pts Select all the intermolecular forces associated with 1-pentanol? H-bonding London Disperson Dipole-dipole Ion-dipole. Your solution’s ready to go! Our Chegg
Chapter 12 Intermolecular Forces Flashcards | Quizlet
On the basis of intermolecular attractions, explain the differences in the boiling points of n-butane (−1 °C) and chloroethane (12 °C), which have similar molar Quizlet
2.12: Intermolecular Forces and Solubilities – Chemistry LibreTexts
The type of intermolecular forces (IMFs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution Chemistry LibreTexts
Chapter 11 Mastering Chemistry Flashcards | Quizlet
Part A Classify each property as associated with a liquid that has strong or weak intermolecular forces. Drag the appropriate items to their respective bins. Part B Quizlet
2.12: Intermolecular Forces – Chemistry LibreTexts
The type of intermolecular forces (IMFs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Because Chemistry LibreTexts
Solved Question 6 3 pt Select all intermolecular forces that – Chegg
See Answer. Question: Question 6 3 pt Select all intermolecular forces that contribute to creating a solution of NaCl in 1- pentanol. H-bonding Dipole-dipole London Disperson lon-dipole. Show transcribed image text. Here’s the best way to solve it. chegg.com
10.1 Intermolecular Forces – Chemistry 2e | OpenStax
Identify the types of intermolecular forces experienced by specific molecules based on their structures; Explain the relation between the intermolecular forces present within OpenStax
Intermolecular Forces – Hydrogen Bonding, Dipole Dipole Interactions – Boiling Point \U0026 Solubility
10.21A | How To Find The Intermolecular Forces In Ch3Ch2Oh (C2H5Oh)
How To Identify Intermolecular Forces?
Intermolecular Forces – Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions
Intermolecular Forces Explained
Ranking Intermolecular Forces – Compare Highest/Lowest Boiling Points With Imf’S
How To Identify The Intermolecular Force A Compound Has: London Dispersion, Dipole Dipole, H-Bonding
Which Intermolecular Force(S) Do The Following Pairs Of Molecules Experience? Pentanol With Another…
What Types Of Intermolecular Forces Exist Between Nh3 And H2O? Select All That Apply. Dipole Induce…
Unit 3.1 – Intermolecular And Interparticle Forces
Link to this article: select all the intermolecular forces associated with 1-pentanol..
See more articles in the same category here: bmxracingthailand.com/what
