What is the structure of chromate and dichromate ion?
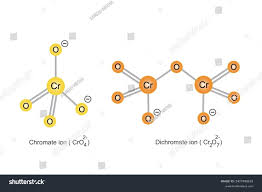
Chromate ion (CrO42–) has a tetrahedral shape. This means the chromium atom (Cr) sits at the center of the structure, and four oxygen atoms (O) are positioned at the corners of a tetrahedron. The bond angles between the oxygen atoms are all 109.5 degrees.
Dichromate ion (Cr2O72–) is a bit more complex. It’s made up of two tetrahedral units joined together by a shared oxygen atom. Each tetrahedral unit has a chromium atom at its center, and it’s bonded to three oxygen atoms. The two tetrahedrons are then linked by a single oxygen atom, creating a Cr-O-Cr bond angle of approximately 126 degrees.
Why the difference in bond angles? The Cr-O-Cr bond angle in dichromate is slightly larger than the O-Cr-O bond angles within the tetrahedrons. This is because of the repulsion between the negatively charged oxygen atoms. The shared oxygen atom is essentially “pulled” away from the chromium atoms, causing a slight expansion in the Cr-O-Cr bond angle.
Let me know if you want to learn more about the chemical properties or reactions involving chromate and dichromate ions!
What is the difference between dichromate and chromate?
Dichromate and chromate are both compounds of chromium, but they differ in their chemical structure and properties. Chromate is a chemical compound containing the chromate ion (CrO42-), while dichromate contains the dichromate ion (Cr2O72-). Chromate is typically used as a corrosion inhibitor in paints and coatings, while dichromate is more commonly used as an oxidizing agent in various industrial processes.
The key difference between chromate and dichromate lies in their ability to inhibit corrosion. Chromate forms a protective layer on the metal surface, preventing corrosion by acting as a barrier against moisture and oxygen. This layer is typically more effective than dichromate, which only serves as a thin sealer.
Dichromate is often used in conjunction with chromate to enhance its corrosion protection properties. However, it’s important to note that both chromate and dichromate can be harmful to human health and the environment. They are classified as hazardous substances, and their use is strictly regulated.
What is the formula for dichromate and chromate ion?
Chromate is an ion with the formula CrO4^2-, while dichromate has the formula Cr2O7^2-. Both are chromium oxyanions with chromium in the +6 oxidation state.
You’ll find these ions in salts, like potassium chromate (K2CrO4) or sodium dichromate (Na2Cr2O7). These compounds are great oxidizing agents, meaning they readily grab electrons from other molecules, causing them to be oxidized. They’re quite powerful in chemistry!
Here’s a little more about the structure and chemistry of chromate and dichromate:
Chromate is tetrahedral, with the chromium atom at the center and four oxygen atoms surrounding it.
Dichromate, on the other hand, has a more complex structure where two chromate units link together through a shared oxygen atom. This forms a linear shape with a central oxygen bridging the two chromium atoms.
The most important thing to remember is that these ions are interconvertible. They can switch back and forth depending on the pH of the solution:
In basic conditions (high pH), chromate is the dominant form.
In acidic conditions (low pH), dichromate is the dominant form.
This interconversion happens through a simple reaction where water molecules get involved. It’s like a dance where the ions rearrange themselves to fit the environment!
This interconversion is essential in various chemical processes, particularly in reactions involving oxidation and reduction. For instance, dichromate is used in various analytical methods, such as titrations, where its oxidizing power is harnessed to determine the concentration of substances.
Understanding the structures and properties of chromate and dichromate is vital for anyone working with these powerful compounds in chemistry and other scientific fields.
What is the shape of chromate ion and its structure?
Let’s dive a bit deeper into the structure of the chromate ion. The central chromium atom has six valence electrons, and each oxygen atom has six valence electrons. To form the chromate ion, the chromium atom shares its six valence electrons with the four oxygen atoms, forming four covalent bonds. Each oxygen atom also carries a negative charge, which is why the overall charge of the chromate ion is -2.
The tetrahedral geometry of the chromate ion can be explained by the VSEPR theory, which stands for Valence Shell Electron Pair Repulsion. This theory states that the electron pairs in the valence shell of an atom will repel each other, and they will arrange themselves in a way that minimizes these repulsions. In the case of the chromate ion, the four oxygen atoms, along with their lone pairs of electrons, will arrange themselves around the central chromium atom in a way that minimizes the repulsion between them, leading to a tetrahedral shape.
The tetrahedral shape of the chromate ion is crucial to its chemical properties. This shape allows the chromate ion to be a good oxidizing agent, as the oxygen atoms are readily available to accept electrons. This property is why chromate ions are often used in corrosion inhibitors, pigments, and tanning agents.
What is the structure of the dichromate ion there is?
Let’s break down the structure of the dichromate ion. Each chromium atom is bonded to four oxygen atoms, three of which are terminal and one bridging. The bridging oxygen atom is shared between the two chromium atoms. This gives the ion a tetrahedral geometry around each chromium atom.
The linear Cr-O-Cr bond is crucial to the stability of the dichromate ion. The shared oxygen atom creates a strong bond between the chromium atoms. This bond also results in a delocalized pi system. This system involves the overlap of the d orbitals of the chromium atoms with the p orbitals of the oxygen atoms. This delocalization contributes significantly to the stability and characteristic color of the dichromate ion.
What is the molecular structure of chromate?
Let’s break down the structure:
Chromium Atom: The chromium atom sits at the center of the tetrahedron. It has a +6 oxidation state, meaning it has lost six electrons.
Oxygen Atoms: Four oxygen atoms surround the chromium atom, each positioned at the corners of the tetrahedron. These oxygen atoms have a -2 charge each.
This arrangement results in a highly symmetrical structure with the oxygen atoms evenly spaced around the central chromium atom. The tetrahedral geometry is a common structure in chemistry, particularly for molecules with a central atom bonded to four other atoms.
This structure is significant because it affects the chemical properties of the chromate ion. For instance, the arrangement of the oxygen atoms influences the reactivity and the way the chromate ion interacts with other molecules. The tetrahedral geometry allows for the formation of strong bonds with other molecules, leading to the chromate ion’s use in various applications, like pigments, corrosion inhibitors, and in the leather tanning process.
What is the difference between K2Cr2O7 and K2CrO4?
The main difference between the two is their color and chemical structure. Potassium chromate is yellow while potassium dichromate is orange.
This difference in color is due to the different oxidation states of the chromium atom. In potassium chromate, chromium is in the +6 oxidation state, while in potassium dichromate, it’s in the +6 oxidation state. The difference in oxidation state affects how the compounds interact with light, resulting in their different colors.
Another interesting difference is that potassium chromate can be formed from potassium dichromate by reacting it with potassium hydroxide. This reaction is shown in the following equation:
K₂Cr₂O₇ + 2KOH → 2K₂CrO₄ + H₂O
This equation shows how potassium dichromate is converted to potassium chromate in the presence of a base like potassium hydroxide.
Here’s a deeper dive into the relationship between potassium chromate and potassium dichromate:
Chromate: The chromate ion (CrO₄²⁻) has a tetrahedral shape and is a powerful oxidizing agent. It’s commonly used in pigments, dyes, and leather tanning.
Dichromate: The dichromate ion (Cr₂O₇²⁻) is formed by the condensation of two chromate ions. The dichromate ion has a linear shape and is a stronger oxidizing agent than the chromate ion. You’ll find it used in various industrial applications, such as electroplating, photography, and organic synthesis.
Interconversion: The equilibrium between chromate and dichromate ions depends on the pH of the solution. In acidic solutions, the equilibrium favors the formation of dichromate, while in basic solutions, it favors the formation of chromate. This is because the addition of a base like potassium hydroxide shifts the equilibrium towards the chromate ion, as explained in the previous chemical equation.
Both potassium chromate and potassium dichromate are strong oxidizing agents and should be handled with caution. They can cause skin and eye irritation, so it’s important to use appropriate personal protective equipment when working with them.
How does chromate turn into dichromate?
Chromate ions (CrO4^2-) and dichromate ions (Cr2O7^2-) exist in an equilibrium in solution. This means they can interconvert depending on the conditions. The equilibrium can be represented by the following equation:
2 CrO4^2- + 2 H+ <=> Cr2O7^2- + H2O
In this equation, adding H+ ions will favor the formation of dichromate ions. This is because the reaction shifts to the right to consume the added H+ ions. Essentially, the addition of H+ ions “pushes” the equilibrium towards the formation of dichromate ions.
We can reverse this process by adding OH- ions. OH- ions react with H+ ions to form water, which reduces the H+ concentration in the solution. This reduction in H+ concentration shifts the equilibrium back to the left, favoring the formation of chromate ions.
In summary, adding H+ ions promotes the formation of dichromate ions from chromate ions. Conversely, adding OH- ions favors the formation of chromate ions from dichromate ions. This interconversion of chromate and dichromate ions is a fundamental aspect of their chemistry and plays a crucial role in various chemical processes.
Which is more stable chromate or dichromate?
Chromate ions are more stable in basic solutions, while dichromate ions are more stable in acidic solutions. This is due to the equilibrium that exists between these two ions.
Here’s a breakdown of the equilibrium:
In basic solutions, the concentration of hydroxide ions (OH-) is high. This favors the formation of chromate ions (CrO4^2-) by removing protons from the dichromate ion (Cr2O7^2-).
In acidic solutions, the concentration of hydrogen ions (H+) is high. This favors the formation of dichromate ions by adding protons to the chromate ion.
Think of it like this: The presence of hydroxide ions in basic solutions “pushes” the equilibrium towards the chromate side, while the presence of hydrogen ions in acidic solutions “pushes” it towards the dichromate side.
This equilibrium is also influenced by the pH of the solution. The pH scale measures the acidity or alkalinity of a solution. A pH of 7 is neutral, values below 7 are acidic, and values above 7 are basic.
You can observe this shift in equilibrium by changing the pH of a solution containing chromate and dichromate ions. For example, if you add acid to a solution containing chromate ions, the solution will turn orange due to the formation of dichromate ions. Similarly, if you add base to a solution containing dichromate ions, the solution will turn yellow due to the formation of chromate ions.
It’s important to remember that chromate and dichromate ions are in equilibrium, meaning they constantly interconvert. The dominant form of the ion depends on the pH of the solution.
See more here: What Is The Difference Between Dichromate And Chromate? | Structure Of Chromate Ion And Dichromate Ion
What is a dichromate ion?
Dichromate is an anion, which means it’s a negatively charged ion, with the chemical formula Cr2O72-.
Dichromate is a powerful oxidizing agent – it loves to take electrons from other substances! This makes it a popular choice in organic chemistry for reactions where you need to add oxygen or remove hydrogen.
Did you know that dichromate can also act as a primary standard solution? This means it’s used in volumetric analysis to determine the concentration of other solutions. It’s a reliable standard because it’s very pure and stable.
But wait, there’s more! Dichromate has a close cousin – the chromate ion (CrO42-). These two ions are like best friends, constantly switching between each other in aqueous solutions. The balance between chromate and dichromate depends on the pH of the solution.
In acidic solutions, dichromate is the dominant form. Dichromate ions are big and bulky, making them more stable in acidic conditions. However, as the solution becomes more alkaline, the dichromate ions can break down into two chromate ions, which are smaller and prefer the alkaline environment.
This interconversion is a neat example of how the pH of a solution can significantly affect the chemical behavior of ions. It’s a classic example of chemical equilibrium – a state where both chromate and dichromate exist in a balanced state, constantly transforming into each other.
You can think of it like a tug-of-war between chromate and dichromate. In acidic conditions, dichromate holds the rope, but as the solution becomes more alkaline, chromate starts to pull, eventually taking over the rope!
Are dichromate ions interconvertible?
Let’s break down why this happens. The interconversion of chromate and dichromate ions is an equilibrium reaction, meaning it’s reversible. The reaction is influenced by factors like pH and the concentration of hydrogen ions (H⁺). Think of it like a seesaw:
On one side, you have chromate ions (CrO₄²⁻). They are favored in basic solutions, where the pH is higher and there are fewer hydrogen ions.
On the other side, you have dichromate ions (Cr₂O₇²⁻). They are favored in acidic solutions, where the pH is lower and there are more hydrogen ions.
The reaction between chromate and dichromate ions looks like this:
2 CrO₄²⁻ (aq) + 2 H⁺ (aq) ⇌ Cr₂O₇²⁻ (aq) + H₂O (l)
This equation shows that two chromate ions react with two hydrogen ions to form one dichromate ion and one water molecule. The reaction is reversible, meaning it can proceed in both directions.
In summary, the interconversion of chromate and dichromate ions is a dynamic process that is influenced by the pH of the solution. Chromate ions are favored in basic solutions, while dichromate ions are favored in acidic solutions. This dynamic equilibrium allows for a fascinating interplay between these two forms of chromium in aqueous environments.
What is the difference between chromium chromate and dichromate?
You’re right to be curious about the difference between chromate and dichromate ions. They are closely related, but their behavior in solution depends on the pH.
Here’s the breakdown:
Chromate (CrO4^2-) ions are stable in alkaline (basic) solutions, while dichromate (Cr2O7^2-) ions are stable in acidic solutions. So the statement “The chromate ion can exist only under acidic conditions while the dichromate ion can exist only in neutral/alkaline conditions” is false.
* The oxidation state of chromium in both chromate and dichromate ions is +6. The difference lies in the way they bond with oxygen. In chromate, chromium is bonded to four oxygen atoms, while in dichromate, it’s linked to seven oxygen atoms through a shared oxygen bridge.
* Both potassium chromate (K2CrO4) and potassium dichromate (K2Cr2O7) are soluble in water.
To understand this interplay between chromate and dichromate, let’s delve deeper:
The key to understanding this shift lies in the equilibrium between the two ions. The reaction is reversible:
2 CrO4^2- (aq) + 2 H+ (aq) ⇌ Cr2O7^2- (aq) + H2O (l)
In acidic conditions, there’s an abundance of hydrogen ions (H+). The equilibrium shifts to the right, favoring the formation of dichromate ions.
Conversely, in alkaline conditions, the concentration of hydrogen ions is low. The equilibrium shifts to the left, favoring the formation of chromate ions.
Think of it like a seesaw. The addition of hydrogen ions tips the scales towards dichromate, while removing them tips the scales towards chromate.
This equilibrium is also affected by temperature. Increasing the temperature generally shifts the equilibrium towards the products, meaning more dichromate is formed.
Understanding this equilibrium is crucial for various applications, including:
Analytical chemistry: The color change from yellow chromate to orange dichromate is used as an indicator in titrations.
Industrial processes: Dichromate is used in the production of pigments, leather tanning, and electroplating.
The dynamic interplay between chromate and dichromate showcases the fascinating chemistry of chromium and its compounds. This simple equilibrium underlies a wide range of applications, highlighting the importance of understanding the relationship between pH and chemical reactions.
What is the equilibrium between chromate and dichromate?
The key players in this chemical ballet are chromate (CrO₄²⁻) and dichromate (Cr₂O₇²⁻) ions. They’re both colorful, with chromate being yellow and dichromate being orange. The equilibrium reaction looks like this:
2CrO₄²⁻ (aq) (yellow) + 2H⁺ (aq) ⇌ Cr₂O₇²⁻ (aq) (orange) + H₂O (l)
This equation tells us that chromate ions can react with hydrogen ions (H⁺) to form dichromate ions and water. The double arrow (⇌) indicates that the reaction can go in both directions. It’s a bit like a seesaw – the reaction can shift to the right or left depending on the conditions.
Think of acid as the conductor of this chemical orchestra. Adding acid to the solution increases the concentration of hydrogen ions, pushing the equilibrium to the right. This means more chromate ions are converted into dichromate ions, leading to a more orange solution.
Here’s a simple analogy to help you visualize this:
Imagine you have a bucket of yellow paint and a bucket of orange paint. If you add a little bit of water to the yellow paint, it’ll stay yellow. But if you add a lot of water, the yellow paint will start to look more orange. That’s because the water is diluting the yellow color. In the case of chromate and dichromate, the hydrogen ions (H⁺) are like the water – they dilute the chromate and make it look more like dichromate.
The equilibrium between chromate and dichromate is an important concept in chemistry. It helps us understand how these ions behave in different solutions and how we can control their relative concentrations. For example, if you want to make a solution with more dichromate ions, you can add acid. Conversely, if you want to make a solution with more chromate ions, you can add a base, which will remove hydrogen ions from the solution and shift the equilibrium to the left.
See more new information: bmxracingthailand.com
Structure Of Chromate Ion And Dichromate Ion: A Detailed Comparison
Hey there, chemistry enthusiasts! Today, we’re taking a deep dive into the fascinating world of chromate and dichromate ions, exploring their structures, properties, and the factors that influence their interconversion. Buckle up, it’s going to be a ride!
A Quick Glance at Chromate and Dichromate Ions
Both chromate (CrO₄²⁻) and dichromate (Cr₂O₇²⁻) ions are polyatomic anions containing chromium in its +6 oxidation state. They’re known for their vibrant colors, which are the result of the d-d electronic transitions in the chromium ion. You’ll often find them in various chemical compounds and solutions, giving those solutions a bright yellow or orange hue.
Chromate Ion: The Building Block
Let’s start with the chromate ion. It’s a tetrahedral structure, just like methane (CH₄). Imagine a central chromium atom surrounded by four oxygen atoms, forming the corners of a tetrahedron.
Here’s a breakdown of its structure:
Chromium (Cr): The central atom in the chromate ion, possessing a +6 oxidation state.
Oxygen (O): Four oxygen atoms surround the chromium atom, each carrying a -2 charge.
The overall charge of the chromate ion is -2, resulting from the sum of the individual charges:
+6 (Cr) + 4(-2) (O) = -2
This arrangement gives the chromate ion a symmetrical and stable structure, accounting for its common occurrence in chemistry.
Dichromate Ion: Two Chromate Ions Unite
Now, let’s move on to the dichromate ion. It’s essentially a dimer of two chromate ions, where one oxygen atom is shared between the two chromium atoms. The dichromate ion, therefore, has a linear structure with two tetrahedral units joined together.
Here’s how to visualize it:
Two chromium (Cr) atoms: They’re at the center of each tetrahedral unit.
Seven oxygen (O) atoms: Six oxygen atoms are bonded to the individual chromium atoms, while one oxygen atom forms a bridge between the two chromium atoms.
The overall charge of the dichromate ion remains -2:
2 (+6) (Cr) + 7 (-2) (O) = -2
The Interplay of pH and Equilibrium
One of the key aspects of chromate and dichromate ions is their interconversion, which depends significantly on the pH of the solution. In acidic solutions, the dichromate ion predominates, while in basic solutions, the chromate ion is more prevalent.
Here’s the equation that governs their equilibrium:
2 CrO₄²⁻ (aq) + 2 H⁺ (aq) ⇌ Cr₂O₇²⁻ (aq) + H₂O (l)
Let’s break down this equation:
Chromate ions (CrO₄²⁻): These ions are favored in basic solutions, where there’s a higher concentration of OH⁻ ions.
Dichromate ions (Cr₂O₇²⁻): These ions are favored in acidic solutions, where there’s a higher concentration of H⁺ ions.
Hydrogen ions (H⁺): These ions react with chromate ions to form dichromate ions, shifting the equilibrium towards the right.
Water (H₂O): Water is a byproduct of the reaction between chromate ions and hydrogen ions.
Applications of Chromate and Dichromate Ions
You might be wondering, “What’s the big deal about these ions? What are they used for?” Well, these ions have a variety of applications in different fields, including:
1. Pigments and Dyes: Chromates and dichromates are essential components in producing various pigments and dyes. Their vibrant colors have made them valuable in paints, textiles, and even some types of glass.
2. Leather Tanning: Dichromate ions play a crucial role in the leather tanning process. They react with the proteins in animal hides, creating cross-links that make the leather more durable and resistant to decay.
3. Chemical Oxidants: The strong oxidizing power of dichromate ions makes them useful as oxidants in various chemical reactions.
4. Analytical Chemistry: Dichromate ions are used in analytical chemistry as titrants for determining the concentration of reducing agents.
5. Corrosion Inhibitors: In certain applications, chromates and dichromates can act as corrosion inhibitors, preventing the rusting of metals.
FAQs
1. Are chromate and dichromate ions toxic?
Yes, both chromate and dichromate ions are highly toxic, especially in high concentrations. They can cause various health problems like skin irritation, respiratory issues, and even kidney damage.
2. How can I distinguish between chromate and dichromate ions in a solution?
You can differentiate between them by observing their color:
Chromate ions: Yellow
Dichromate ions: Orange
By adjusting the pH, you can shift the equilibrium between chromate and dichromate ions, changing the color of the solution.
3. Are there any other compounds that contain chromium in the +6 oxidation state?
Yes, several other compounds contain chromium in the +6 oxidation state, including chromyl chloride (CrO₂Cl₂) and potassium permanganate (KMnO₄).
4. Why is chromium in the +6 oxidation state so important in these ions?
Chromium’s +6 oxidation state allows it to form strong bonds with oxygen atoms, leading to the formation of these stable and highly reactive ions.
5. What are some safety precautions to take when handling chromate and dichromate ions?
Always wear appropriate protective gear, such as gloves, goggles, and a lab coat, when handling chromate and dichromate ions. Avoid contact with skin and eyes. Work in a well-ventilated area to minimize inhalation of any fumes.
6. Are chromate and dichromate ions used in any everyday products?
Yes, chromate and dichromate ions can be found in some everyday products, such as paints, inks, and some types of plastics. However, their use is becoming increasingly restricted due to their toxicity.
7. Is there any research being done to find safer alternatives to chromate and dichromate ions?
Yes, there is ongoing research into developing safer and more sustainable alternatives to chromate and dichromate ions, especially for industrial applications.
8. How can I learn more about the chemistry of chromate and dichromate ions?
You can explore various resources, including textbooks, scientific journals, and online platforms like Khan Academy and Coursera.
9. What is the difference between chromate and dichromate in terms of their oxidizing ability?
Dichromate ions are stronger oxidizing agents than chromate ions. This is because the dichromate ion contains two chromium atoms in the +6 oxidation state, making it more likely to accept electrons and oxidize other substances.
10. What is the role of chromate and dichromate ions in environmental pollution?
Chromate and dichromate ions are known pollutants that can contaminate water sources and soil. They can be released into the environment from industrial processes, such as metal plating and tanning.
Conclusion:
We’ve taken a deep dive into the structure, properties, and applications of chromate and dichromate ions. Understanding their chemistry is crucial for various fields, from industrial processes to environmental protection. As we continue to learn more about these fascinating ions, we can better leverage their benefits while minimizing their potential risks.
(Cr2O72-) – Dichromate Structure, Properties and Uses
Dichromate is an anion with the chemical formula Cr2O72-. It is used as a strong oxidising agent in organic chemistry as well as a primary standard solution in BYJU’S
Structure Of Chromate Ion And Dichromate Ion – YouTube
Structure Of Chromate Ion And Dichromate Ion Video Lecture from D and F Block Elements Chapter of Chemistry Class 12 for HSC, IIT JEE, CBSE & YouTube
Chromate (CrO42-) – Structure, Molecular Mass,
Chromates are the salts of chromic acid which contains the chromate anion with the chemical formula CrO42– and usually have an intense yellow color. Chromate is BYJU’S
A chromate–dichromate equilibrium | Experiment
Try this class practical to investigate an equilibrium between chromate (VI), dichromate (VI) and hydrogen ions. In this experiment, students add dilute sulfuric acid to an aqueous solution of potassium chromate (VI). RSC Education
Chemistry of Chromium – Chemistry LibreTexts
It includes: reactions of chromium (III) ions in solution (summarised from elsewhere on the site); the interconversion of the various oxidation states of chromium; Chemistry LibreTexts
What is the Difference Between Chromate and Dichromate
The chromate ion is the predominant species in alkaline solutions, but dichromate can become the predominant ion in acidic solutions. How are chromate Pediaa.Com
Structure of Chromate and Dichromate ions AND How to
1 watching now Premiere in progress. Started 9 minutes ago. Hello This video will help you understand the structural representation of chromate and YouTube
Chromate Ion Formula & Structure – Purdue University
Learn about the chromate ion, its formula and structure, and how it is used in various chemical reactions. This webpage from Purdue University provides a clear and concise Purdue Chemistry
Draw the structures of chromate and dichromate ions. – Toppr
The sum of the total number of bonds between chromium and oxygen atoms in chromate and dichromate ions is _____. Toppr
Structure Of Chromate Ion And Dichromate Ion – D And F Block Elements – Chemistry Class 12
Structure Of Chromate And Dichomate Ion
How To Draw The Lewis Dot Structure For Cro4 2- (Chromate Ion)
Structure Of Chromate Ion, Structure Of Dichromate Ion
Structure Of Chromate And Dichromate,
Structure Of Chromate And Dichromate Ions
Describe The Structure Of Chromate And Dichromate Ion
Structure Of Chromate \U0026 Dichromate |Part 57|Unit-8 | Cbse Grade 12|D,F Block
Structure Of Chromate (Cro4 2-)And Dichromate (Cr2O7 2-) Ions And How To Intercovert Them.
Metal Complexes 7. Chromate – Dichromate Equilibrium
Link to this article: structure of chromate ion and dichromate ion.
See more articles in the same category here: bmxracingthailand.com/what
