What is the hydrophilic end of a surfactant?
One end of a surfactant molecule is hydrophilic, which means it loves water. This end is attracted to water molecules and easily dissolves in them. The other end of the surfactant is lipophilic, meaning it loves oil. This end dissolves in oily substances like grease or fat.
Because of this unique structure, surfactants can act as a go-between for water and oil. They can help to mix these two substances that normally don’t want to hang out together.
Let’s break down the hydrophilic end in more detail. It’s usually made up of a group of atoms that have a positive or negative charge. This charge allows the hydrophilic end to form strong attractions with water molecules, which are also polar. These attractions are called hydrogen bonds and they are responsible for the hydrophilic end’s love affair with water.
Think of it this way: Imagine you have a group of magnets, with some being positive and some being negative. They are all attracted to each other, but only the positive ones will stick to other positive ones, and only the negative ones will stick to other negative ones. The same goes for water molecules. They have a slightly positive side and a slightly negative side. So, the hydrophilic end of a surfactant, with its positive or negative charge, can easily form bonds with the water molecules.
The hydrophilic end is crucial for how surfactants work. It allows them to form a stable layer at the interface between water and oil, effectively reducing surface tension and making it easier for the two substances to mix. This is why surfactants are used in so many products, from detergents and soaps to shampoos and cosmetics!
What is the hydrophilic end of a surfactant molecule considered to be attracting?
The head of a surfactant molecule is hydrophilic, meaning it loves water. This end is usually made up of a charged group, like a sulfate or phosphate. These charged groups can form strong bonds with water molecules, which are also polar (meaning they have a slightly positive and slightly negative end).
The tail of the surfactant molecule is hydrophobic, meaning it avoids water. This end is typically made up of a long chain of carbon and hydrogen atoms, which are non-polar (meaning they have no charge separation). These tails prefer to associate with other non-polar molecules, like grease and dirt.
This special ability to interact with both water and oil makes surfactants extremely useful in a variety of applications. For example, surfactants are used in detergents to break down grease and dirt so they can be easily washed away. They’re also used in shampoos and body washes to help remove oil and dirt from our skin and hair.
Let’s break down how surfactants do their magic:
Imagine a drop of oil on the surface of water. The oil molecules are hydrophobic, so they don’t want to mix with the water molecules. This creates a barrier between the two substances.
Now, add a surfactant. The hydrophilic heads of the surfactant molecules will stick to the water molecules, while the hydrophobic tails will stick to the oil molecules. This forms a bridge between the water and oil, allowing the oil to break down into smaller droplets.
The surfactant molecules essentially surround the oil droplets, forming tiny “packages” that can then be dispersed throughout the water. These packages are called micelles.
Essentially, surfactants act like tiny emulsifiers, helping to mix substances that wouldn’t normally mix together. This is why they are so important in many everyday products, from cleaning supplies to cosmetics.
What is the tail of a surfactant molecule?
While there are many different types of hydrophilic heads, the hydrophobic tails of surfactants are often very similar. This means the properties of the hydrophobic tail don’t usually determine how a surfactant behaves. It’s the hydrophilic head that’s the key to understanding the function of a surfactant.
Let’s break down the types of hydrophobic tails in more detail:
Hydrocarbon tails are the most common type of hydrophobic tail. They are made up of chains of carbon and hydrogen atoms. Examples of surfactants with hydrocarbon tails include soaps and detergents.
Fluorocarbon tails are made up of chains of carbon and fluorine atoms. They are often used in surfactants that are resistant to oil and grease.
Siloxane tails are made up of chains of silicon and oxygen atoms. They are often used in surfactants that are used in silicone-based products, such as cosmetics and sealants.
The hydrophobic tail of a surfactant is essential for its function. It allows the surfactant to interact with oily or greasy substances and helps to break them down or disperse them in water. Think of it like a bridge between the water-loving hydrophilic head and the oil-loving hydrophobic tail.
What is the lipophilic tail of a surfactant molecule considered to be?
Think of it like this: Imagine a group of people at a party. Some love to chat and mingle (the hydrophilic head of a surfactant), while others prefer to stay in their own groups (the hydrophobic tail). The hydrophobic tails of surfactant molecules are similar to these partygoers who prefer to stay with their own kind. They avoid water and instead bond with each other.
This bonding is crucial for the function of surfactants. Surfactants work by lowering the surface tension of liquids, allowing them to mix more easily. The hydrophobic tails play a key role in this process.
Here’s how it works: When a surfactant is added to water, the hydrophilic heads of the molecules face the water, while the hydrophobic tails cluster together, forming a barrier between the water and whatever the surfactant is trying to mix in. This barrier allows the surfactant to lower the surface tension of the water, allowing it to mix more easily with substances that are normally repelled by it.
Think of it like trying to mix oil and water. Oil and water naturally separate because oil is hydrophobic (water fearing) and water is hydrophilic (water loving). But by adding a surfactant, you can create a bridge between these two substances. The hydrophilic heads of the surfactant molecules will interact with the water, while the hydrophobic tails will interact with the oil, allowing the two substances to mix.
Surfactants are found in a wide variety of products, including soaps, detergents, shampoos, and cosmetics. They play an important role in our daily lives, helping us to clean, wash, and care for our bodies and belongings.
What is a hydrophilic end?
One end of each phospholipid molecule is polar, meaning it has a partial electric charge. This is because it’s made up of a phosphate group, which is a negatively charged molecule. Water is also polar and has electrically charged ends. This means that water is attracted to the oppositely charged end of a phospholipid molecule. We call this end of the phospholipid molecule hydrophilic, which means “water loving.”
Think of it like magnets. The opposite poles of a magnet attract each other. The hydrophilic end of the phospholipid molecule is like the north pole of a magnet, while the water molecule is like the south pole. They attract each other because of their opposite charges.
Because of this attraction, hydrophilic ends are drawn towards water. This is why phospholipids spontaneously form bilayers in water. The hydrophilic ends face the water on both the inside and outside of the cell membrane, while the hydrophobic ends (which don’t like water) face each other in the middle of the membrane. This structure is essential for the formation of cell membranes, which are vital for the functioning of all living cells.
So, to sum it up, a hydrophilic end is simply the part of a phospholipid molecule that loves water! It’s attracted to water because of its partial electric charge, and this attraction plays a crucial role in the formation of cell membranes.
What is the hydrophilic end of a soap molecule?
Soap molecules have two distinct ends:
The hydrophilic head is the part of the molecule that loves water. It’s like a little magnet that attracts water molecules.
The hydrophobic tail is the opposite – it hates water! This part of the molecule prefers to hang out with oil and grease.
Think of it like this: the hydrophilic head is a friendly, outgoing person who loves to be around people (water molecules), while the hydrophilic tail is a shy person who prefers to be alone (with oil and grease).
Now, when you add soap to water, the hydrophilic heads of the soap molecules eagerly jump into the water, forming a circle around the hydrophobic tails. The hydrophobic tails then go looking for dirt and grease, which they’re much happier to be around.
This process is called micelle formation. Imagine a bunch of soap molecules forming a sphere with their hydrophilic heads facing outward and their hydrophobic tails tucked inside. These spheres are like little bubbles that trap the dirt and grease inside, lifting them away from the surface you’re cleaning.
The hydrophilic head is the key to how soap cleans. It’s the part of the molecule that allows soap to mix with water and effectively wash away grease and dirt!
What do hydrophilic molecules attract?
A great example of a hydrophilic molecule is sugar. If you put a spoonful of sugar in your tea, it dissolves and mixes with the water because it’s hydrophilic.
You’ll find hydrophilic molecules at work in many places, including:
Plants: Plants rely on hydrophilic surfaces to absorb water from the soil and transport it throughout their bodies.
Animals: Some animals, like the Schedorhinotermes termite, use hydrophilic surfaces on their bodies and wings to cling to plants. This helps them colonize and live on their chosen plants.
Our bodies: Our cells are also full of hydrophilic molecules that help regulate the flow of water in and out of them. These molecules are crucial for maintaining a healthy balance of fluids in our bodies.
What makes hydrophilic molecules so attracted to water? It’s all about how water molecules are structured. Each water molecule has a slightly positive end and a slightly negative end. This allows them to form hydrogen bonds with other water molecules, which create a strong attraction. Hydrophilic molecules also have a positive or negative charge, which allows them to form similar bonds with water molecules.
This attraction between water and hydrophilic molecules is crucial for many biological and chemical processes. It helps to dissolve substances, transport nutrients, and maintain the proper balance of fluids in our bodies.
What are the hydrophilic and hydrophobic parts of surfactant?
Think of it this way: Imagine a tiny magnet with one end that loves water (the hydrophilic head) and the other end that hates water (the hydrophobic tail). The hydrophobic tail will stick to things like oil or grease, which are also water-hating. This is why surfactants are so good at cleaning!
Let’s break down what’s happening:
Hydrophobic tails: The tails are usually long chains of carbon and hydrogen atoms. These chains are attracted to other non-polar molecules like oils and greases, forming a bond that keeps them together. The tail is repelled by water because water molecules are polar, meaning they have a positive and negative end.
Hydrophilic heads: The heads are usually charged groups like sulfate, phosphate, or carboxylate ions. These heads are attracted to water molecules because they are also polar. The heads form a strong bond with water, creating a layer of water around the heads.
This unique structure allows surfactants to act as a bridge between water and oil or grease. The tails can surround and trap the oil or grease, while the heads interact with the water. This allows the water to pull the oil or grease away from the surface it’s stuck on, effectively cleaning it.
What are the two ends of surfactants?
The hydrophilic head is attracted to water. Think of it as the “water-loving” part of the molecule. This end is usually made up of charged or polar groups, like a phosphate group or a sulfate group. These groups can interact with water molecules through hydrogen bonding.
The hydrophobic tail is repelled by water. It’s the “water-hating” part of the molecule. This end is typically composed of a long chain of hydrocarbons. These chains are non-polar and can’t form hydrogen bonds with water. Instead, they prefer to interact with other non-polar molecules like oils and fats.
So, how does this unique structure make surfactants so useful?
Surfactants act as bridges between water and oil. The hydrophilic head sticks into the water while the hydrophobic tail sticks into the oil or grease. This allows surfactants to break up oil droplets into smaller droplets, which are then dispersed throughout the water. This is why surfactants are used in so many cleaning products, like detergents and soaps. They help to remove dirt and grease from surfaces.
Here’s an analogy: Imagine you have a bunch of marbles (representing oil droplets) in a bucket of water. You want to mix them together but they just sit there in clumps. Now, add some dish soap (which contains surfactants) to the bucket. The surfactants attach themselves to the marbles, with their heads sticking into the water and their tails sticking into the marbles. This creates a barrier around each marble, preventing them from sticking together. The result is a mixture of water and oil droplets that are evenly dispersed.
Are surfactants hydrophilic or hydrophobic?
Think of a surfactant molecule as having two personalities. One part of the molecule is hydrophilic (“water-loving”), which means it attracts water. The other part is hydrophobic (“water-hating”) and is drawn to oil or grease. This dual nature is what makes surfactants so special.
Let’s break down the science a bit. Polar molecules are hydrophilic, meaning they have a positive and a negative end. Think of a magnet with a north and south pole – that’s a good analogy. On the other hand, nonpolar molecules lack this charge separation and are hydrophobic. They prefer to hang out with other nonpolar molecules.
Now, back to surfactants. Because they have both a hydrophilic and hydrophobic end, they are called amphiphilic. This makes them great at bridging the gap between water and oil. They can help to mix substances that wouldn’t normally blend together, like oil and water.
Imagine you’re trying to wash your hands with oily grime. Soap, a type of surfactant, comes to the rescue! The hydrophobic end of the soap molecule attaches itself to the oil on your hands, while the hydrophilic end sticks to the water. This allows the oil to be lifted away from your skin and into the water, leaving your hands clean.
Surfactants are truly remarkable molecules! They play a crucial role in many everyday products, from detergents and shampoos to food additives and even pharmaceuticals. Their ability to interact with both water and oil makes them indispensable for a wide variety of applications.
Which structure forms when surfactant molecules aggregate in a liquid?
Think of a surfactant molecule like a little creature with two personalities: a hydrophilic head that loves water and a hydrophobic tail that hates it. When these surfactants are in water, they get together and form a circle, with all the hydrophobic tails pointing inward and the hydrophilic heads facing outward towards the water. This circle is called a micelle.
Imagine you’re at a pool party, and you’re surrounded by water-loving people. But there’s one shy person who doesn’t like water. To make them comfortable, everyone forms a circle around them, with their backs to the water and their faces toward the shy person. This is kind of like how micelles form.
Micelles are important because they help surfactants do their job, which is to reduce the surface tension of liquids. This means they make it easier for liquids to spread out and mix together.
Let’s break down why micelles are so important in various applications:
Cleaning: Think about how dish soap gets rid of grease. The hydrophobic tails of the surfactants in dish soap attach to the grease molecules, while the hydrophilic heads stick out into the water. This forms a micelle that traps the grease and allows it to be washed away.
Cosmetics:Surfactants in shampoos and body washes form micelles to remove dirt and oil from your skin and hair. They also help to create a smooth, creamy texture in these products.
Food: Surfactants in ice cream and other foods help to stabilize the mixture and prevent the ingredients from separating. They also help to improve the texture and mouthfeel of these products.
Medicine: Some surfactants are used in medicines to help the body absorb certain drugs or to deliver them to specific areas of the body.
So, the next time you see a micelle, think of it as a tiny, water-loving circle, helping to make life a little bit cleaner, smoother, and tastier!
What is a surfactant molecule?
Surfactant molecules are special compounds that have a unique ability to interact with both water and oil. Imagine them as tiny little bridges connecting two different worlds!
These molecules have two distinct parts:
A hydrophilic head: This part loves water and is attracted to it. Think of it as the friendly, social part of the molecule that wants to be surrounded by water molecules.
A hydrophobic chain: This part is afraid of water and prefers to hang out with oil or other fatty substances. It’s like the shy, introverted part of the molecule that wants to avoid water at all costs.
This special structure allows surfactant molecules to do some amazing things. They can form micelles, which are tiny spheres where the hydrophobic chains cluster together in the center, shielded from water by the hydrophilic heads that face outwards. These micelles are like little pockets that can trap oil or grease molecules, making it easier to wash them away.
Let’s take a closer look at these two parts of a surfactant molecule:
Hydrophilic Head:
* This part is usually made up of polar groups, which have a positive and a negative end.
* These groups can form hydrogen bonds with water molecules, making the head soluble in water.
* Common examples of hydrophilic heads include:
Sulfonate groups (-SO3-)
Phosphate groups (-PO4-)
Ammonium groups (-NH3+)
Carboxylate groups (-COO-)
Hydrophobic Chain:
* This part is usually made up of non-polar groups, which have no distinct positive or negative ends.
* These groups cannot form hydrogen bonds with water molecules, making the chain insoluble in water.
* Common examples of hydrophobic chains include:
Long hydrocarbon chains (like those found in fatty acids)
Alkyl groups (like those found in detergents)
So, in essence, surfactant molecules are tiny little mediators that help bridge the gap between water and oil. Their unique structure allows them to break down oil and grease, making it easier to remove them from surfaces. This is why surfactants are so important in many everyday products, like soap, shampoo, and detergents.
What is the true nature of surfactants?
Micelles are tiny spheres that emerge when surfactants are introduced to a solution. Imagine each surfactant molecule as a tiny two-faced creature, with a hydrophilic head that loves water and a hydrophobic tail that shuns it. In a water-based solution, the hydrophilic heads of the surfactants naturally turn towards the water, forming a shell around the micelle, while the hydrophobic tails huddle together in the center, creating a water-repellent core.
This clever arrangement allows surfactants to play a crucial role in a wide range of applications. For instance, in detergents, surfactants create micelles that trap grease and dirt, making it easier to wash clothes. In cosmetics, surfactants help to emulsify oils and water, allowing the creation of smooth, creamy lotions.
The formation of micelles is a dynamic process influenced by factors like the concentration of surfactants, temperature, and the presence of other molecules. As the concentration of surfactants increases, more micelles form, and they can even interact with each other, creating complex structures. This intricate dance of micelles is what gives surfactants their remarkable properties, making them essential players in a multitude of applications.
See more new information: bmxracingthailand.com
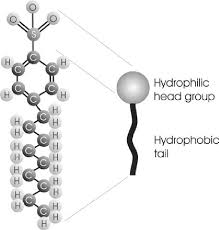
The Hydrophilic End Of A Surfactant Molecule Is Considered The: Head Group
The Hydrophilic End of a Surfactant Molecule: The Head of the Operation
You know how oil and water don’t mix? That’s because oil is hydrophobic (water-fearing), and water is hydrophilic (water-loving). Surfactants are like the peacekeepers in this water-oil conflict. They’re special molecules designed to bridge the gap between these two worlds.
Think of a surfactant molecule as having two distinct parts:
1. The hydrophilic head: This end of the molecule is attracted to water. It’s like the social butterfly of the molecule, happy to mingle with water molecules.
2. The hydrophobic tail: This end of the molecule is attracted to oil and grease. It’s the shy one, preferring to hang out with its oily buddies.
So, what’s the hydrophilic end of a surfactant molecule considered? It’s considered the polar head.
The Polar Head: A Water Magnet
Remember that water is a polar molecule. That means it has a slightly positive end and a slightly negative end. The hydrophilic end of a surfactant molecule is also polar, with a similar positive and negative charge distribution. This makes them besties – they’re both attracted to each other, forming strong bonds.
Why is the Polar Head Important?
Think about washing your dishes. You need soap to get rid of the greasy residue, right? That’s where surfactants come in. They work by breaking down the oily gunk on your dishes, allowing water to wash it away.
Here’s how it works:
1. The hydrophobic tail of the surfactant molecule attaches itself to the grease on your dishes.
2. The hydrophilic head of the surfactant molecule, being attracted to water, points outward towards the surrounding water.
3. This forms a micelle – a sphere-shaped structure where the hydrophobic tails are tucked inside, shielded from water, and the hydrophilic heads are on the outside, interacting with the water.
Think of it like a bunch of tiny little balls, with the greasy dirt trapped inside, and the water-loving parts of the surfactant molecules facing outward, holding onto the water.
The micelles then get washed away with the rinse water, carrying the grease with them.
The Hydrophilic Head: A Versatile Player
The polar head of a surfactant molecule isn’t just good at cleaning dishes. It’s a multi-talented molecule with a wide range of applications, including:
* Detergents: Surfactants are the key ingredients in detergents, making them powerful cleaning agents.
* Emulsifiers: They can help create stable mixtures of oil and water, like in mayonnaise or milk.
* Foaming agents: They are used to create foam in products like shampoo, shaving cream, and fire extinguishers.
* Wetting agents: They can help water spread out and wet surfaces more easily, like in pesticides and paints.
Understanding the Hydrophilic End: Key to Surfactant Functionality
The hydrophilic end of a surfactant molecule is the key to its functionality. It allows the surfactant to interact with water, forming micelles that can trap and remove dirt, grease, and other unwanted substances. It’s the reason why surfactants are so important in our daily lives, from cleaning our homes to making our food taste better.
Let’s Sum it Up
So, in short, the hydrophilic end of a surfactant molecule is considered the polar head. It’s the water-loving part of the molecule that makes it so effective at cleaning, emulsifying, foaming, and wetting.
FAQs
What are some examples of surfactants?
There are many different types of surfactants, but some common examples include:
* Sodium dodecyl sulfate (SDS): A common surfactant found in detergents, shampoos, and toothpastes.
* Sodium lauryl sulfate (SLS): Another popular surfactant found in many personal care products.
* Tween 80: A non-ionic surfactant used in food, pharmaceuticals, and cosmetics.
* Span 80: A non-ionic surfactant used in cosmetics and pharmaceuticals.
What are the different types of surfactant heads?
Surfactant heads can be categorized into different types based on their chemical structure and charge:
* Anionic surfactants: These have a negatively charged head group, such as SDS.
* Cationic surfactants: These have a positively charged head group.
* Non-ionic surfactants: These have no charge on their head group, such as Tween 80 and Span 80.
* Zwitterionic surfactants: These have both a positive and negative charge on their head group.
How are surfactants used in everyday life?
Surfactants are all around us! Here are some examples:
* Cleaning products: Detergents, dish soap, hand soap, laundry detergent.
* Personal care products: Shampoos, conditioners, body wash, toothpaste.
* Food products: Mayonnaise, ice cream, salad dressings.
* Pharmaceuticals: Drug delivery systems, antiseptics.
* Agriculture: Pesticides, herbicides.
* Industrial applications: Textile processing, oil recovery.
Hopefully, this gives you a better understanding of the hydrophilic end of a surfactant molecule and how it plays a vital role in so many aspects of our lives. If you have any further questions, feel free to ask!
Structure and Applications of Surfactants | IntechOpen
Surfactant molecules have two parts, a lipophilic (apolar) part that retains fat and a hydrophilic (polar) part that is miscible with water. The lipophilic portion consists of one or more aliphatic, straight or branched or aromatic or even alkylaromatic hydro- or IntechOpen
Chapter 15 scalp Flashcards | Quizlet
The _ of a surfactant molecule is water attracting. Lipophilic ends. The _ of a surfactant molecule is oil- attracting. Study with Quizlet and memorize flashcards Quizlet
Micelle Definition, Structure, and Function – Science Notes and
In an inverted micelle, the hydrophilic heads of the surfactant molecules orient inward towards the core, while the hydrophobic tails face outward towards the Science Notes and Projects
Lesson Surfactants: Helping Molecules Get Along
A micelle is organized such that the hydrophilic heads of the surfactant molecule are on its surface while the hydrophobic tails of the surfactant molecule point towards its center. TeachEngineering
Surfactants | SpringerLink
Surface-active agents (abbreviated surfactants) are amphiphilic molecules consisting of a lyophobic (hydrophobic when in water) and lyophilic (hydrophilic when in water) Springer
What Are Surfactants | School of Chemistry
Surfactants are amphiphilic molecules with both hydrophilic and hydrophobic groups. The hydrophilic end of a surfactant molecule is the headgroup that interacts with water, while the hydrophobic end is the tail University of Bristol
Surfactant Molecule | SpringerLink
Surface-active agents (usually referred to as surfactants) are amphipathic molecules consisting of a nonpolar hydrophobic portion, usually a straight or branched hydrocarbon Springer
Introduction to Surfactants – ScienceDirect
Hydrophilicity and Hydrophobicity of Surfactant Molecules. The hydrophilic functional group of surfactant molecules strongly prefers interaction with polar entities ScienceDirect
1.20.2: Surfactants and Micelles – Chemistry LibreTexts
In the context of aqueous solutions, surfactant molecules are amphipathic meaning that the solutes have dual characteristics : amphi ≡ dual and pathi ≡ sympathy. Chemistry LibreTexts
Episode 2: Surfactant Chemistry
How Soap Works – 3D Animation
How Detergents Works: Surfactants
Surfactant
Surfactants – Cosmetic Science In 300 Seconds
Surfactant And Surface Tension In Respiration | Breathing Mechanics | Respiratory Physiology
Combatting The Coronavirus With Surfactants
What Are Surfactants \U0026 Micelles – Chemistry Of Surfactants
Soap Micelles Formation – Science
How Does Shampoo Work?? #Shorts
Link to this article: the hydrophilic end of a surfactant molecule is considered the:.
See more articles in the same category here: bmxracingthailand.com/what
