Let’s discuss the question: the lewis structure of n2h2 shows. We summarize all relevant answers in section Q&A of website Bmxracingthailand.com in category: Blog technology. See more related questions in the comments below.
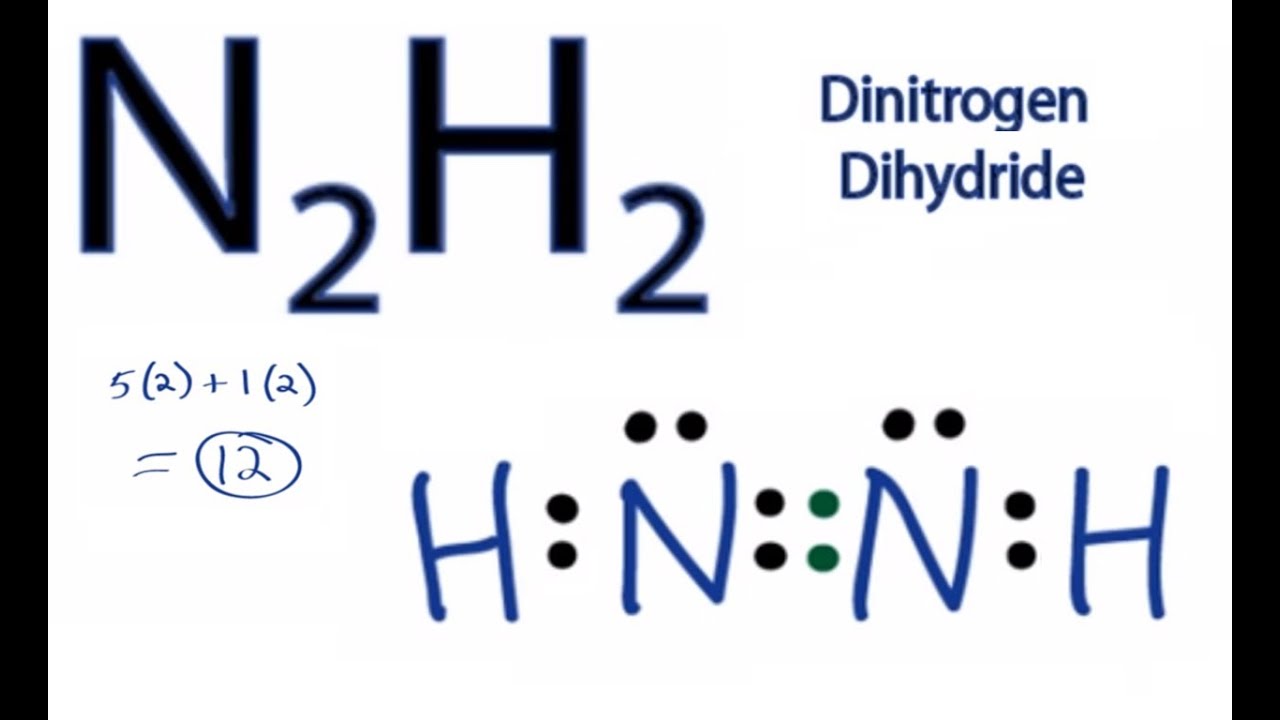
How many valence electrons does n2h2 have?
In the Lewis structure for N2H2 there are a total of 12 valence electrons. In order to complete the octets on the Nitrogen (N) atoms you will need to form a double bond between the N atoms.
How many valence electrons does ch3ch2oh have?
For, ethanol, total pairs of electrons are ten in their valence shells.
N2H2 Lewis Structure: How to Draw the Lewis Structure for Dinitrogen dihydride
Images related to the topicN2H2 Lewis Structure: How to Draw the Lewis Structure for Dinitrogen dihydride
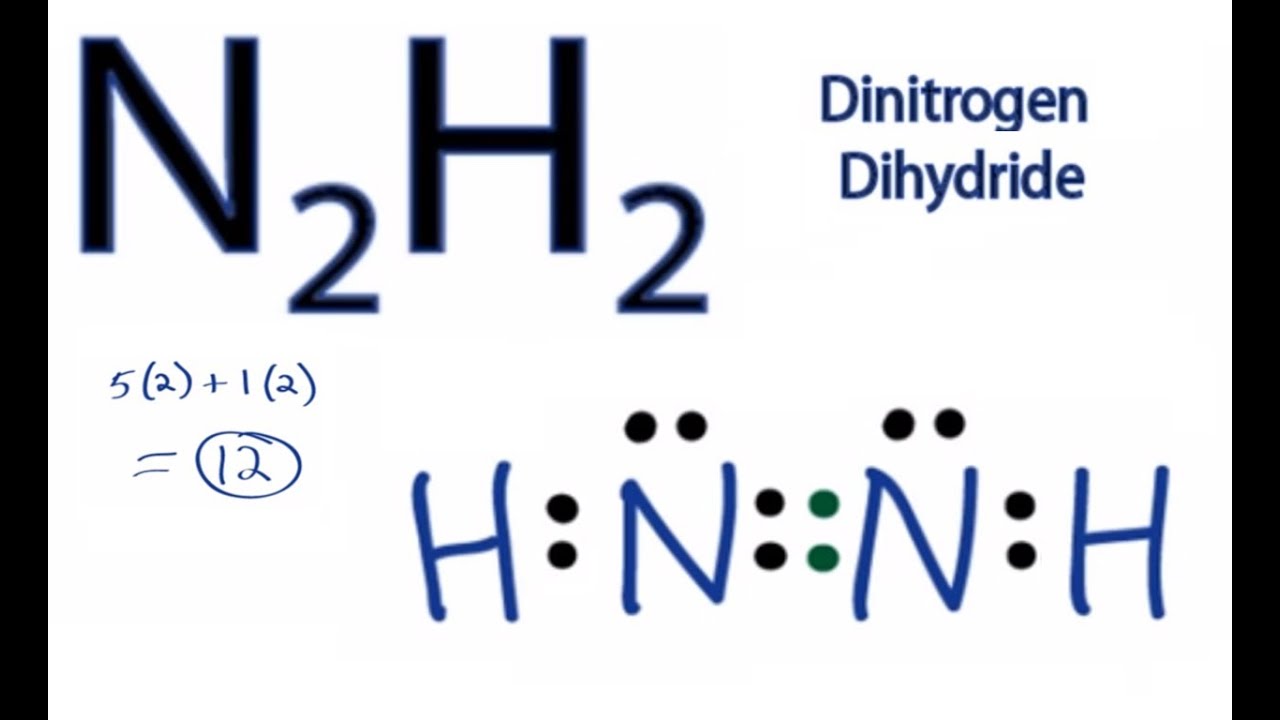
How many valence electrons does CH3CH2Cl have?
The number of valence electrons in CH3CH2Cl is: 14.
What type of compound is N2H2?
Diazene is a nitrogen hydride. It is a conjugate acid of a diazenide.
What type of intermolecular force is CH3CH2OH?
But, CH3CH2OH alone exhibits hydrogen bonding, which is much stronger than the intermolecular forces that are active in the other choices. The boiling points of the hydrogen halides are listed below.
What is the functional group of CH3CH2OH?
Alcohol is a functional group which is bonded to alkyl or aryl chains. The structure of the alcohol functional group is $ R-OH $ . Similarly the given compound $ C{{H}_{3}}C{{H}_{2}}OH $ is ethanol, i.e. alcoholic functional group.
What is the Iupac name of CH3CH2OH?
Ethanol | CH3CH2OH – PubChem.
What is the molecular shape of CHClO CHClO?
The shape of CHClO, or chloroformaldehyde (common name formyl chloride), is trigonal planar (see the figure below).
What is the Iupac name of CHCl3?
Which Lewis structure is possible for N2O?
The most stable Lewis structure of N2O is represented by option (D). In this structure, more electronegative O atom bears negative charge and less electronegative N atom bears a positive charge. Hence, the charge separation that is as that predicted by electronegativity.
N2H2 Lewis Structure (Dinitrogen Dihydride)
Images related to the topicN2H2 Lewis Structure (Dinitrogen Dihydride)

How do resonance structures differ?
Two resonance structures differ in the position of multiple bonds and non bonding electron. The placement of atoms and single bonds always stays the same.
How many valence electrons are in the Lewis structure of PO43?
In the Lewis structure of PO43- there are a total of 32 valence electrons. For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Remember, PO4 3- has a negative three charge on the molecule.
What is the hybridization of N2H2?
In the molecule of N2H2, there are two sigma bonds, one pi bond and a lone pair of electrons around the nitrogen atom. Here again, the lone pair can be considered equivalent to a sigma bond. Hence, the nitrogen atom is sp2 hybridized.
Is N2H2 ionic or covalent?
The covalent compound shown is diazene. It contains 2 central nitrogen atoms that are bonded to each other and to 2 hydrogen atoms.
Is N2H2 polar or nonpolar?
Answer and Explanation: The molecule is polar. The molecule N2H2 N 2 H 2 is made up of two elements: nitrogen (N) and hydrogen (H).
What is N2H2 used for?
Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion.
Is diazene linear or planar?
Would you expect diazine to be a linear molecule (all four atoms on the same line)? Would you expect the molecule to be planar (all four atoms in the same plane)? A Th l l i b th li d l A. The molecule is both linear and planar.
Is diazene a compound or mixture?
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, E (trans) and Z (cis).
What intermolecular forces are present in CH3CH2CH2CH3?
CH3CH3 and CH3CH2CH2CH3 have weak dispersion forces only, so have the lowest boiling points. CH3CH2CH2CH3 has more atoms, so more dispersion forces and hence the higher boiling point of the two.
N2H2 Molecular Geometry, Bond Angles (and Electron Geometry)
Images related to the topicN2H2 Molecular Geometry, Bond Angles (and Electron Geometry)

Does CH3CH2OH have dispersion forces?
Only dispersion forces are present and these are largest in the longer chain alkane as it has more electrons (more bonds). Both molecules possess dipole moments but CH3CH2OH contains hydrogen bonded to an electronegative element so H-bonding is possible.
Is CH3CH2OH a hydrogen bond?
CH3CH2OH is completely soluble in water. It can form hydrogen bonds with water, and its hydrocarbon section is small.
Related searches
- how many electrons are in the lewis structure of a nitrite ion no2
- what does a lewis structure show
- the lewis structure of hcn shows that
- the ability of an atom in a molecule to attract electrons is best quantified by the
- there are covalent bonds in the lewis structure of ch3ch2cl
- what shape is n2h2
- the lewis structure of the co32 ion is
- the lewis structure of the co32- ion is ________.
- resonance structures differ by
- what does the lewis structure of n2h2 show
- what is the lewis structure for n2h2
- there are ______ covalent bonds in the lewis structure of ch3ch2cl.
- the lewis structure of n2h2 shows chegg
- the lewis structure of n2h2 shows quizlet
- the correct lewis structure of n2h2 shows
- which of the following is the best lewis structure for n2h2
Information related to the topic the lewis structure of n2h2 shows
Here are the search results of the thread the lewis structure of n2h2 shows from Bing. You can read more if you want.
You have just come across an article on the topic the lewis structure of n2h2 shows. If you found this article useful, please share it. Thank you very much.
