What is the purpose of adding pentavalent impurity atom to a silicon crystal?
Imagine silicon, the backbone of our electronic world. By itself, it’s a pretty good conductor, but we can make it even better by adding a special ingredient: pentavalent impurities.
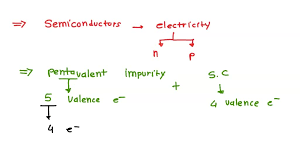
These impurities, like phosphorus or arsenic, are like tiny superheroes for silicon. They have five valence electrons, compared to silicon’s four. When we introduce these impurities into the silicon crystal, they share four of their electrons with the surrounding silicon atoms, forming strong bonds.
But what about that extra, fifth electron? It’s not tied down by the bonds and becomes free to roam around the crystal. These free electrons act like tiny messengers, carrying electrical current throughout the silicon, making it a much more efficient conductor.
That’s why pentavalent impurities are called “donor atoms”. They generously donate these free electrons, giving silicon a boost in conductivity. This process, known as n-type doping, is crucial for creating semiconductors that form the foundation of our modern electronic devices.
Think of it like adding a few extra lanes to a highway. The more lanes you have, the more cars (or electrons) can flow through, making the traffic (or current) move faster. This is exactly what happens when we add pentavalent impurities to silicon – it creates more “lanes” for electrons to flow, resulting in increased conductivity.
The ability to control conductivity with impurities is a fundamental concept in semiconductor technology. By carefully controlling the type and concentration of impurities, we can tailor the properties of silicon and create a wide range of electronic devices, from transistors to integrated circuits, all of which form the foundation of our digital world.
What is pentavalent impurity or donor?
You know how semiconductors are super cool because they can be manipulated to conduct electricity better, right? Well, pentavalent impurities are like little helpers that make semiconductors even more awesome.
Imagine a semiconductor with a crystal structure made of silicon atoms, each with four valence electrons. Now, picture a pentavalent impurity, like phosphorous, entering this crystal structure. Phosphorus has five valence electrons. When it takes the place of a silicon atom, four of its electrons form bonds with neighboring silicon atoms, just like the silicon atoms. But that fifth electron doesn’t have anywhere to go. It’s like an extra guest at a party with no chair!
This extra, unbound electron is what makes phosphorous a donor impurity. It’s essentially free to roam around within the semiconductor crystal, making it easier for electricity to flow. Pentavalent impurities are like tiny electron donors, increasing the number of free electrons in the semiconductor and making it more conductive. Think of it like adding extra lanes to a highway – it makes traffic (electricity) move faster!
Now, let’s get a little more technical. The extra electron from the pentavalent impurity is not completely free. It’s actually bound to the donor impurity atom, creating what’s called a donor energy level just below the conduction band. This means that a small amount of energy is needed to excite the electron to the conduction band, where it can freely move and contribute to conductivity.
This process of adding pentavalent impurities to a semiconductor is called n-type doping. The “n” stands for negative, because the added electrons make the semiconductor more negatively charged. So, you can see why pentavalent impurities are sometimes called donor impurities – they “donate” free electrons to the semiconductor, making it more conductive.
What happens when a pentavalent impurity is added to a pure semiconductor?
Imagine a pure semiconductor, like silicon. Silicon has four valence electrons, meaning it can form four bonds with other silicon atoms. When you add a pentavalent impurity, like phosphorus, which has five valence electrons, something interesting happens. Four of the phosphorus’s valence electrons form bonds with the surrounding silicon atoms, just like the silicon atoms themselves. But the fifth electron is left over, becoming a free electron. These free electrons can easily move throughout the semiconductor, increasing its conductivity.
This is why we call it an n-type semiconductor: the “n” stands for “negative,” representing the excess of negatively charged electrons. Essentially, doping a semiconductor with a pentavalent impurity increases the number of free electrons, making the semiconductor more conductive. It’s like adding a few drops of water to a bucket of sand – the sand becomes more mobile and less resistant to flow. In the same way, adding a pentavalent impurity makes the semiconductor more conductive, allowing it to carry an electrical current more easily.
Why is pentavalent known as a donor atom?
Let’s break down why this happens. In a pure semiconductor, like silicon, each atom has four valence electrons, forming covalent bonds with its neighboring atoms. However, when a pentavalent atom, like phosphorus, is introduced, it has five valence electrons. Four of these electrons bond with the silicon atoms, but the fifth electron is loosely bound and readily available for conduction. This extra electron increases the conductivity of the semiconductor.
Think of it like this: imagine a group of people sharing four cookies, with each person having one. If someone brings an extra cookie, they can share it with someone who doesn’t have one, increasing the number of cookies being shared. In the same way, the extra electron from the pentavalent atom becomes available for sharing, increasing the conductivity of the semiconductor.
Here’s an example:
Silicon (Si) has four valence electrons, forming covalent bonds with four surrounding silicon atoms.
Phosphorus (P) has five valence electrons. It forms four covalent bonds with four silicon atoms, but the fifth electron is loosely bound and easily donated.
* This donated electron becomes a free electron, increasing the conductivity of the silicon material.
This process of adding pentavalent impurities is called n-type doping, as it creates an excess of negative charge carriers (electrons) in the semiconductor.
What is the purpose of the pentavalent impurity?
Think of it like this: Silicon, with four valence electrons, needs four electrons to form stable bonds with its neighboring atoms. Pentavalent impurities, with their extra electron, readily give up that electron, filling the “hole” in the silicon lattice. This extra electron becomes free to move around, contributing to the electrical conductivity of the semiconductor.
This process is like injecting a bunch of extra players into a soccer game. The silicon crystal, like a team with just enough players, becomes more dynamic and conductive with the addition of these extra electrons. This is why pentavalent impurities are also known as donor impurities – they generously donate free electrons to the semiconductor. These free electrons can then carry electrical current, making the semiconductor more conductive.
Why do we add only trivalent and pentavalent impurities?
Let’s break this down a bit more:
Trivalent impurities have three valence electrons, which means they have one fewer electron than the four required to form covalent bonds with neighboring atoms in the semiconductor crystal. This creates a “hole” where an electron is missing, and this “hole” can act as a positive charge carrier.
Pentavalent impurities have five valence electrons, one more than needed to bond with the neighboring atoms. This extra electron is easily freed, becoming a negative charge carrier.
So, by adding trivalent or pentavalent impurities, we essentially create either “holes” or extra free electrons, which in turn increase the semiconductor’s conductivity. This process is called doping, and it’s a fundamental technique for controlling the electrical properties of semiconductors, allowing us to build essential components like transistors, diodes, and integrated circuits.
Why a donor impurity is introduced to a pure semiconductor?
Think of it like this: Imagine you have a group of friends playing a game of tag, but they’re all wearing the same color shirts, making it hard to tell who’s who. Now, let’s say one friend arrives wearing a bright red shirt. That red shirt stands out, and it’s easier for the other friends to spot and tag them. Similarly, a donor impurity acts like that red shirt. It sticks out and allows for easier electron flow.
The presence of donor impurities doesn’t simply increase electron concentration, it also leads to a decrease in the hole concentration. Remember that in a pure semiconductor, there’s an equal number of electrons and holes. When donor impurities are added, they contribute extra electrons. These extra electrons recombine with the existing holes, reducing their number. This is why donor impurities are called n-type dopants – they increase the concentration of electrons, making the semiconductor n-type.
The balance between electrons and holes is crucial in determining the semiconductor’s conductivity. By introducing donor impurities, we can control this balance and create semiconductors with specific electrical properties. This allows us to create all sorts of useful devices like transistors and diodes.
What are pentavalent vs trivalent impurities?
Pentavalent impurities are atoms with five valence electrons, like arsenic (As) or phosphorus (P). When these impurities are added to a semiconductor like silicon (Si), they donate an extra electron to the crystal lattice. This extra electron is free to move, creating a n-type semiconductor – “n” stands for “negative” because the free electrons carry a negative charge.
Trivalent impurities, on the other hand, have three valence electrons, such as boron (B) or gallium (Ga). When added to silicon, they create a “hole” or electron deficiency in the lattice. This “hole” acts like a positive charge carrier, leading to the creation of a p-type semiconductor – “p” for “positive.”
Think of it like this: in a n-type semiconductor, the extra electrons are like tiny, negative marbles that can move freely. In a p-type semiconductor, the “holes” are like empty spaces in a box, and the positive charge carriers are like marbles that can jump into those empty spaces.
Both pentavalent and trivalent impurities are essential for creating the electronic properties of semiconductors that make them so useful in devices like transistors, diodes, and integrated circuits.
What is pentavalent impurity p-type semiconductor?
Think of it this way: p-type semiconductors are created when you add a tiny amount of an element with five valence electrons (like arsenic, antimony, or bismuth) to a pure semiconductor like silicon or germanium. These elements have one more valence electron than the semiconductor they’re added to.
Here’s the key: when these extra electrons are added, they create “holes” in the semiconductor’s structure. These holes act like positive charges, which is why it’s called a p-type semiconductor (p for positive). These holes can move freely through the semiconductor, allowing it to conduct electricity.
So, the trivalent impurities you mentioned are actually used to create n-type semiconductors, where the added element has three valence electrons. This creates an excess of free electrons in the semiconductor, leading to an n-type semiconductor (n for negative).
Let me give you an analogy to help solidify this concept. Imagine a parking lot full of cars. If you add an extra car (a pentavalent impurity), it creates a space where a car could be parked but isn’t. That “empty space” is similar to a hole in a p-type semiconductor. Now, if you take away a car from the parking lot (like a trivalent impurity), it creates an extra parking space. This is similar to the extra electrons created in an n-type semiconductor.
Understanding the difference between n-type and p-type semiconductors is crucial for comprehending how transistors and other semiconductor devices work.
See more here: What Is Pentavalent Impurity Or Donor? | The Purpose Of A Pentavalent Impurity Is To
See more new information: bmxracingthailand.com
The Purpose Of A Pentavalent Impurity Is To | What Is The Purpose Of Adding Pentavalent Impurity Atom To A Silicon Crystal?
You see, semiconductors are like the middle children of the element world – not quite a conductor like copper, not quite an insulator like rubber. They’re this sweet spot that allows us to control the flow of electricity, making them the building blocks of all sorts of cool tech like computers, smartphones, and even your fancy new electric car.
But semiconductors in their pure form aren’t always the most cooperative. They need a little push, a little nudge to become truly useful. That’s where pentavalent impurities come in.
Think of it like this: a semiconductor is like a highway with lots of lanes but no cars. It’s capable of carrying lots of traffic, but it needs a bit of a boost to get things moving.
Now, imagine you introduce a few pentavalent impurities. These are like tiny little cars that are really good at carrying electrons. They’re like the VIPs of the electron world, and they know exactly where to go.
The reason they’re called pentavalent is because they have five valence electrons – you know, those outer electrons that are involved in chemical bonding. This is different from the semiconductor, which typically has four valence electrons.
So, when you add these pentavalent impurities, they donate one of their extra electrons to the semiconductor, increasing its conductivity. It’s like suddenly the highway is filled with cars, and they’re all cruising along smoothly.
N-Type Semiconductors: The Result of Pentavalent Impurities
The process of adding pentavalent impurities is called doping, and it’s like giving the semiconductor a little bit of a personality. By adding these extra electrons, we create what’s called an n-type semiconductor.
Now, why n-type? Because these extra electrons have a negative charge. You can think of them like tiny little magnets, and they make the semiconductor negatively charged.
Why N-Type Semiconductors are So Important
N-type semiconductors are like the backbone of the electronics world. They allow us to control the flow of electricity in a very specific way, which is crucial for building all sorts of devices.
For example, you can’t have a transistor without an n-type semiconductor. Transistors are like the tiny switches that control the flow of electricity in our electronic devices.
Imagine a transistor as a door with a gate. The n-type semiconductor is the door, and it can be opened or closed by applying a small voltage to the gate. This control over the flow of electricity is what makes electronics possible!
Examples of Pentavalent Impurities
Let’s talk about some of the most common pentavalent impurities used in semiconductor manufacturing:
Phosphorus (P): One of the most common and popular impurities used to create n-type semiconductors.
Arsenic (As): A powerful dopant, often used for high-speed applications.
Antimony (Sb): Similar to arsenic, it’s another popular choice for high-speed semiconductors.
Key Takeaways
To recap, pentavalent impurities are like little superheroes for semiconductors. They increase conductivity, leading to n-type semiconductors, which are essential for building transistors and other electronic components.
They’re a bit like magic ingredients, allowing us to turn a basic semiconductor into a powerful tool for creating the technologies we use every day.
FAQs
Now, let’s address some common questions about pentavalent impurities:
Q: How are pentavalent impurities added to semiconductors?
A: The process of adding pentavalent impurities to semiconductors is called doping. This is usually done during the crystal growth process of the semiconductor material. Think of it like adding a pinch of spice to your dish, but instead of flavor, we’re adding conductivity.
Q: Are there different types of impurities besides pentavalent impurities?
A: You bet! There are trivalent impurities, which have three valence electrons. They do the opposite of pentavalent impurities, creating p-type semiconductors, which have an excess of holes (positive charge carriers). Think of them like the opposite of those extra electrons, like little positive magnets.
Q: What happens when you combine n-type and p-type semiconductors?
A: That’s when things get really interesting! When you combine n-type and p-type semiconductors, you create a p-n junction. These junctions are the heart of many electronic devices, like diodes, transistors, and solar cells.
Q: What are some other applications of n-type semiconductors?
A: You can find n-type semiconductors everywhere in electronics! They are used in:
Transistors: As I mentioned earlier, they’re the building blocks for modern electronics.
Integrated circuits (ICs): The brains of your computers and smartphones, packed with millions of transistors.
Diodes: Like one-way streets for electrons, they control the flow of electricity in one direction.
Light-emitting diodes (LEDs): They turn electricity into light, making our lives brighter.
Q: What are the challenges of using pentavalent impurities?
A: While pentavalent impurities are super helpful, there are a few challenges:
Purity: To ensure good performance, the semiconductors must be very pure. Even a tiny amount of impurities can drastically affect conductivity.
Doping concentration: You need to be precise with the amount of impurities you add. Too little, and you don’t get the desired effect. Too much, and you can actually reduce conductivity.
Temperature: The temperature at which you dope the semiconductor can also impact its properties.
Q: Is it possible to remove pentavalent impurities from a semiconductor?
A: It’s not impossible, but it’s a complex process. There are techniques like annealing that can reduce the concentration of impurities, but it can be difficult to completely eliminate them.
Q: What’s the future of pentavalent impurities in semiconductor technology?
A: Pentavalent impurities are still playing a crucial role in semiconductor technology and will continue to be important for years to come. Researchers are constantly exploring new and innovative ways to use pentavalent impurities to create even more efficient and powerful semiconductors.
This is just a glimpse into the fascinating world of pentavalent impurities. These little guys may be small, but they have a big impact on our technological world. Hopefully, this exploration has given you a better understanding of their role in making our devices work.
Solved The purpose of a pentavalent impurity is to: create – Chegg
The purpose of a pentavalent impurity is to: create minority carriers increase the number of holes increase the number of free electrons O reduce the conductivity of silicon. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to Chegg
What are trivalent and pentavalent impurities? – Answers
When trivalent atoms are added to intrinsic semiconductors, the resulting material is called a p-type material. When pentavalent impurity atoms are used, the Answers
1.4: Doped Materials – Engineering LibreTexts
N-type material is created by adding pentavalent impurities, that is, a dopant with five electrons in its outer shell. Examples include phosphorus, arsenic and Engineering LibreTexts
Doping (semiconductor) – Wikipedia
In semiconductor production, doping is the intentional introduction of impurities into an intrinsic (undoped) semiconductor for the purpose of modulating its electrical, optical and structural properties. Wikipedia
9.7: Semiconductors and Doping – Physics LibreTexts
An impurity with an extra electron is known as a donor impurity, and the doped semiconductor is called an n-type semiconductor because the primary carriers of Physics LibreTexts
The purpose of a pentavalent impurity is to (a) reduce the c – Quizlet
Find step-by-step Engineering solutions and your answer to the following textbook question: The purpose of a pentavalent impurity is to (a) reduce the conductivity of silicon (b) Quizlet
Extrinsic Semiconductors – Definition, Types and
When a pentavalent impurity is added to a pure semiconductor, an n-type semiconductor is obtained. This is because a pure semiconductor has 4 valence electrons. When a pentavalent impurity is added, one electron BYJU’S
Semiconductor Theory – Electronics-Lab.com
Doping of intrinsic semiconductors with pentavalent impurity atoms makes electrons as majority carriers. A property of N-type semiconductor. In N-type semiconductor, impurity is the donor of electrons and has a bound Electronics-Lab.com
The Purpose Of Pentavalent Impurity Is To: Increase The Number Of Free Electron Reduce The Conducti…
Classification Of Semiconductors (Intrinsic/Extrinsic, P-Type/N-Type)
Extrinsic Semiconductors
What Is Pentavalent And Trivelent Impurities
What Is Intrinsic And Extrinsic Semiconductors | What Is Doping | Electronic Devices \U0026 Circuits
Ictp Colloquium On \”Cosmology And Unification\”
8. Multielectron Atoms And Electron Configurations
Determining Electronic Configuration Of Atoms \U0026 Ions | Class 11 Chemistry | 2024 Federal Board
Link to this article: the purpose of a pentavalent impurity is to.
See more articles in the same category here: https://bmxracingthailand.com/what
