Where do electrons flow in a cell?
In an electrolytic cell, electrical energy is transformed into chemical energy. Electrons flow from the anode to the cathode through an external power supply. This movement of electrons drives the chemical reactions happening inside the cell. It’s important to note that only ions move within the solution, not electrons.
Imagine the anode like a positive pole attracting negative ions. These negative ions give up their electrons at the anode, which then travel through the external circuit to the cathode. The cathode acts like a negative pole attracting positive ions, which then receive electrons from the circuit. This transfer of electrons powers the chemical reactions taking place within the cell, creating a new product.
Here’s a simplified way to think about it:
Anode: Where oxidation occurs, and electrons are released.
Cathode: Where reduction occurs, and electrons are gained.
This flow of electrons from the anode to the cathode through the external circuit is what drives the chemical reactions in an electrolytic cell, essentially turning electrical energy into chemical energy.
Where do the electrons flow in a voltaic cell from?
Electrons always flow from the anode to the cathode. This means they move from the site of oxidation (where a substance loses electrons) to the site of reduction (where a substance gains electrons).
You can also think of it this way: electrons flow from the oxidation half-cell to the reduction half-cell. In terms of the standard electrode potential (Eocell) of the half reactions, electrons will flow from the more negative half reaction to the more positive half reaction.
To understand why this happens, imagine a tug-of-war. The more negative half-reaction is like the team that’s losing the battle – they’re more likely to “give up” electrons. The more positive half-reaction is like the team winning – they’re more likely to “grab” those electrons.
Let’s delve deeper into why electrons flow from anode to cathode:
Think of a voltaic cell as a little battery, creating electrical energy from a chemical reaction. The anode is where oxidation occurs – a substance loses electrons. These electrons, now free, are looking for a place to go. The cathode, on the other hand, is where reduction happens. A substance there is ready to accept electrons.
The difference in electrical potential between the anode and cathode creates a driving force – like a pressure difference in a pipe. The electrons are pushed from the anode (high electron potential) to the cathode (low electron potential). This flow of electrons constitutes the electrical current that powers our devices.
A good way to visualize this is to think of a river flowing downhill. The anode is the high point, the cathode is the low point, and the electrons are like the water flowing from the higher elevation to the lower elevation.
Here’s another analogy. Imagine you have two buckets, one filled with water and the other empty. The water-filled bucket represents the anode (high electron potential), and the empty bucket represents the cathode (low electron potential). If you connect the two buckets with a pipe, the water will flow from the full bucket to the empty bucket. Similarly, electrons flow from the anode (where there’s an excess of electrons) to the cathode (where there’s a deficiency of electrons).
Understanding the direction of electron flow is crucial for comprehending how voltaic cells work. It’s the heart of how these cells generate electricity!
Do electrons flow into anode or cathode?
Let’s break down the concept of electron flow in electrochemical cells.
Electrochemical cells are devices that convert chemical energy into electrical energy (galvanic cells) or electrical energy into chemical energy (electrolytic cells).
* The anode is the electrode where oxidation occurs. Oxidation is the loss of electrons.
* The cathode is the electrode where reduction occurs. Reduction is the gain of electrons.
Therefore, because electrons are lost at the anode and gained at the cathode, electrons always flow from the anode to the cathode. This flow is driven by the potential difference between the electrodes, which is determined by the specific chemical reactions occurring at each electrode.
Now, let’s talk about electrolytic cells specifically. In electrolytic cells, the chemical reaction is not spontaneous and requires an external power source to drive the reaction. The external power source provides the energy needed to force electrons to flow from the anode to the cathode. This means that the anode is positively charged and the cathode is negatively charged. This setup pushes the electrons against the natural flow, causing a non-spontaneous reaction to occur.
Think of it this way: you’re pushing a rock uphill. It’s not going to roll up on its own, you need to apply force to make it go against gravity. In an electrolytic cell, the external power source is the force that pushes the electrons uphill, so to speak, causing the non-spontaneous reaction to happen.
This is important to understand because it highlights the key difference between galvanic cells and electrolytic cells. In galvanic cells, the reaction is spontaneous and generates electricity. The electron flow is from the anode to the cathode because the chemical reaction drives this flow. In electrolytic cells, the reaction is non-spontaneous, requiring an external power source to drive the electron flow from the anode to the cathode.
Where do the electrons flow in a galvanic cell?
The anode is the electrode where oxidation occurs, meaning that electrons are lost. The cathode is the electrode where reduction occurs, meaning that electrons are gained. Since electrons always flow from a region of high concentration to a region of low concentration, they naturally move from the anode, where there is an abundance of electrons due to oxidation, to the cathode, where there is a deficiency of electrons due to reduction.
This movement of electrons through the external circuit creates an electric current. This current can be used to power devices, such as light bulbs or motors.
To understand the flow of electrons in a galvanic cell, it is helpful to think about the analogy of a water wheel. In a water wheel, water flows from a higher elevation to a lower elevation, turning the wheel in the process. In a galvanic cell, electrons flow from the anode, which has a higher potential energy, to the cathode, which has a lower potential energy, creating an electrical current in the process.
The direction of electron flow in a galvanic cell is determined by the difference in potential energy between the anode and the cathode. The greater the difference in potential energy, the stronger the driving force for electron flow and the higher the current generated.
Why do electrons flow from Zn to Cu?
Since electrons are negatively charged, they move from the zinc electrode, which now has a negative charge, towards the copper electrode, which now has a positive charge. This is why zinc becomes the negative terminal and copper becomes the positive terminal of the cell.
To understand this better, let’s break down the concept of reactivity and its connection to electron flow:
Reactivity: Reactivity refers to how readily an element undergoes a chemical reaction, particularly the tendency to lose or gain electrons. More reactive elements like zinc tend to lose electrons easily.
Electron Flow: Electrons always flow from a region of higher electron density (where there are more free electrons) to a region of lower electron density (where there are fewer free electrons). This is a fundamental principle in electrochemistry.
In our zinc-copper cell, zinc has a higher tendency to lose electrons, meaning it has a higher electron density. Copper has a lower tendency to lose electrons, meaning it has a lower electron density. When connected, the electrons naturally flow from the higher electron density region (zinc) to the lower electron density region (copper). This flow of electrons creates the electric current we observe in the circuit.
What is the direction of current flow in a galvanic cell?
Let’s break it down:
Cathode: This is where reduction occurs. Reduction is a chemical process that involves the gain of electrons. So, the cathode is where electrons are consumed.
Anode: This is where oxidation occurs. Oxidation is a chemical process that involves the loss of electrons. The anode is where electrons are released.
Since electrons flow from the anode (where they are released) to the cathode (where they are consumed), the conventional current (which we think of as the flow of positive charge) is in the opposite direction: from the cathode to the anode.
Imagine a river flowing downhill. The water (electrons) flows from the higher elevation (anode) to the lower elevation (cathode). The current, however, is considered to flow *upstream* from the cathode to the anode. This is just a convention, but it’s important to remember that the actual flow of electrons is from the anode to the cathode.
Here’s a more detailed explanation to help you visualize the process:
Electron flow: In a galvanic cell, the electrons released during oxidation at the anode flow through an external circuit to the cathode. This circuit could be a wire, a light bulb, or any other electrical component. The electrons flow because they are attracted to the positive charge on the cathode.
Conventional current flow: Even though the electrons flow from the anode to the cathode, we conventionally represent the current flow as the movement of positive charges. This is because the direction of the current is defined as the direction that positive charges would flow if they were present. So, the conventional current flows from the cathode to the anode.
Electrolyte: The movement of ions within the electrolyte solution in the galvanic cell completes the circuit. The electrolyte contains ions that are attracted to the electrodes with opposite charges. This movement of ions maintains electrical neutrality in the cell.
So, while the electrons flow from the anode to the cathode, the conventional current flow in a galvanic cell is from the cathode to the anode. This is a key concept to understand the operation of these electrochemical cells.
See more here: Where Do Electrons Flow In A Cell? | Where Do Electrons Flow In A Galvanic Cell
How do electrons flow through a galvanic cell?
The electrons travel through an external circuit to the copper electrode. Here, the Cu²⁺ (aq) ions in contact with the Cu electrode accept these electrons and become Cu (s). Since Cu²⁺ is reduced, the Cu electrode is the cathode. So, in a galvanic cell, electrons flow from anode to cathode through an external circuit.
Imagine the copper electrode as a welcoming destination for the electrons. They journey through the external circuit, driven by the difference in electrical potential between the two electrodes. This potential difference is like a force pushing the electrons along. When they reach the copper electrode, they meet up with copper ions (Cu²⁺) that are happily waiting to accept them. These ions are positively charged, so they’re attracted to the negatively charged electrons.
The electrons combine with the copper ions, causing them to transform into neutral copper atoms (Cu). This process is called reduction, where the copper ions gain electrons and become less positively charged. The copper electrode is the cathode because it’s where reduction occurs.
Think of it like a bustling airport. The electrons are like travelers arriving at the copper electrode, the cathode, ready to be welcomed and transformed. They’re on a mission to find their perfect match – the copper ions – and they’re eager to make the journey from the anode to the cathode.
How is current produced in a galvanic cell?
In a galvanic cell, electrons flow externally through the circuit from the anode to the cathode. This happens because there’s a difference in potential energy between the two electrodes. Think of it like a water flowing downhill, seeking a lower energy state. The anode is the electrode where oxidation occurs, meaning it loses electrons, while the cathode is where reduction occurs, meaning it gains electrons.
Here’s a more detailed explanation:
Oxidation at the anode releases electrons, and these electrons want to move to a lower energy state.
* The electrons flow through an external circuit (a wire), driven by the difference in potential energy between the anode and the cathode.
* This external flow of electrons is what we call electric current.
* At the cathode, the electrons are accepted by the ions in the electrolyte solution, completing the circuit and driving the chemical reaction.
To sum it up, the difference in potential energy between the anode and cathode, caused by the chemical reactions occurring at each electrode, drives the flow of electrons through the external circuit, resulting in electric current.
How does a galvanic cell work?
Electrons flow through the external conductor, which is what makes galvanic cells useful. You can think of it as a little electric current. This flow is driven by the electromotive force (EMF) of the cell. The EMF is a measure of how easily the two electrodes dissolve into the electrolyte.
Think of it like this: Imagine you have two different metals, one that really wants to dissolve and give up its electrons, and another that’s a bit more hesitant. When you put them in an electrolyte, the eager metal will shed electrons and become positively charged. Those electrons flow through the external circuit to the more hesitant metal, making it negatively charged. This difference in charge creates a potential difference, or the EMF.
Here’s the thing: the EMF is a bit like a “push” that forces the electrons to move through the external circuit. The stronger the push, the more electrons flow. This flow of electrons is what we harness to do work, like power a light bulb or run a motor.
The key is that the EMF is determined by the half-cell potentials of the two electrodes. Each electrode has a unique tendency to dissolve in the electrolyte. The difference in these tendencies creates the EMF. So, to create a stronger current, you need a bigger difference in their tendencies to dissolve!
In simpler terms, you need to find two metals that are very different in their eagerness to give up electrons. That difference is what creates the driving force for the electrons to flow. This flow is what makes galvanic cells so useful for generating electricity.
How do electrons flow from anode to cathode in a galvanic cell?
In a galvanic cell, electrons always flow from the anode to the cathode through an external circuit. Think of it like a tiny electric current! This happens because the anode is where oxidation takes place, meaning it loses electrons. Meanwhile, the cathode is where reduction occurs, meaning it gains electrons.
Picture this: the anode is like a generous friend who’s always willing to share their electrons, while the cathode is like a hungry friend eager to receive those electrons. This exchange of electrons creates an electric current that can be harnessed to power things, just like a battery!
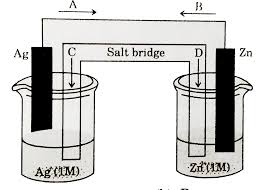
Now, let’s take a look at the Daniell cell, a classic example of a galvanic cell. The Daniell cell features two half-cells – one containing a zinc electrode immersed in a zinc sulfate solution and the other containing a copper electrode submerged in a copper sulfate solution.
Here’s how it works:
Zinc is more reactive than copper, meaning it has a stronger tendency to lose electrons. So, at the anode, zinc atoms lose electrons and become zinc ions (Zn²⁺), entering the solution. These electrons then travel through the external circuit towards the cathode.
* At the cathode, copper ions (Cu²⁺) in the solution accept the incoming electrons and become copper atoms, plating onto the copper electrode.
This continuous flow of electrons from anode to cathode creates a steady electric current, making the Daniell cell a powerful source of energy.
To further understand the flow of electrons in a galvanic cell, think of it as a “tug-of-war” between the anode and cathode. The anode wants to get rid of its electrons, while the cathode eagerly awaits their arrival. This “tug-of-war” results in a continuous flow of electrons from the anode to the cathode, creating the electric current that powers our devices.
See more new information: bmxracingthailand.com
Where Do Electrons Flow In A Galvanic Cell?
We’ll explore the inner workings of these electrochemical powerhouses, dissecting the flow of electrons, the role of the electrodes, and the electrolyte.
Ready? Let’s get started!
The Electron Flow in a Galvanic Cell
Imagine a galvanic cell as a tiny electrical factory. It uses the power of chemical reactions to generate electricity.
Think of a battery; that’s a common example of a galvanic cell. Inside a galvanic cell, there are two key players:
Electrodes: These are the conductive materials that act as the gateway for electrons to enter and leave the cell. They’re typically made of metals or graphite.
Electrolyte: This is the solution or paste that allows ions to flow between the electrodes. Think of it as the “highway” for charged particles.
Now, let’s get to the heart of the matter: where do the electrons flow?
Here’s the deal:
1. The Anode: The anode is the electrode where oxidation happens. This means that the metal in the anode loses electrons and forms positively charged ions. These ions enter the electrolyte.
2. The Cathode: The cathode is the electrode where reduction happens. This means that positively charged ions from the electrolyte gain electrons and form a neutral metal.
3. The Electron Flow: The electrons released from the anode don’t just vanish into thin air! They travel through an external circuit – think of this as a wire connecting the two electrodes – to reach the cathode. This is the electron flow we’re talking about.
Essentially, the electrons are pushed away from the anode, where they’re in excess, and are attracted to the cathode, where they’re needed to complete the reduction process.
A Deeper Dive into the Process
Let’s take a closer look at how this electron flow is maintained within a galvanic cell:
The Salt Bridge: A salt bridge is often used in galvanic cells to maintain electrical neutrality. It’s a special bridge filled with a solution containing ions, like potassium chloride (KCl).
Maintaining Neutrality: The salt bridge allows negative ions from the electrolyte to migrate to the anode and positive ions to the cathode. This movement of ions balances the charge buildup that would otherwise occur and stop the electron flow.
Without this balancing act, the cell would quickly become too charged and the chemical reactions driving the electron flow would grind to a halt.
Real-World Examples:
Let’s consider a familiar example: a zinc-copper galvanic cell.
1. Zinc Electrode: The zinc electrode acts as the anode. It undergoes oxidation, losing electrons and forming zinc ions (Zn²⁺) which dissolve into the electrolyte.
2. Copper Electrode: The copper electrode acts as the cathode. It undergoes reduction as copper ions (Cu²⁺) from the electrolyte gain electrons and deposit as solid copper metal on the copper electrode.
3. Electron Flow: The electrons released from the zinc electrode flow through an external circuit to the copper electrode, powering a light bulb or any other device connected to the circuit.
FAQs:
Here are some frequently asked questions about the electron flow in a galvanic cell:
Q: What’s the direction of electron flow in a galvanic cell?
A: Electrons flow from the anode (where oxidation occurs) to the cathode (where reduction occurs) through an external circuit.
Q: Can you explain the difference between an electrolytic cell and a galvanic cell?
A: The main difference lies in the direction of electron flow and the type of energy involved. In a galvanic cell, electrons flow spontaneously from the anode to the cathode, generating electrical energy from chemical reactions. In an electrolytic cell, electrons are forced to flow from the cathode to the anode, consuming electrical energy to drive non-spontaneous chemical reactions.
Q: What is the purpose of the electrolyte in a galvanic cell?
A: The electrolyte serves as a medium for the movement of ions, allowing the completion of the redox reactions and maintaining electrical neutrality. It enables the flow of charge throughout the cell.
Q: What are some applications of galvanic cells in everyday life?
A: Galvanic cells are used in various applications, including:
Batteries: From your phone to your car, batteries power many devices.
Corrosion Prevention: Galvanic cells can be used to protect metals from corrosion.
Electroplating: They’re essential in coating metal surfaces with other metals.
Q: What factors affect the electron flow in a galvanic cell?
A: Several factors influence the electron flow in a galvanic cell, including:
The nature of the electrodes: The type of metal used for the electrodes affects the voltage and the rate of electron flow.
The concentration of the electrolyte: Higher electrolyte concentration often leads to a faster electron flow.
The temperature: Increased temperature generally accelerates the rate of chemical reactions and electron flow.
Remember: The electron flow in a galvanic cell is driven by the chemical reactions happening at the electrodes. It’s a fascinating dance of electrons, ions, and the potential difference that powers our world!
How do electrons flow in a galvanic cell? | Socratic
Electrons flow from the anode to the cathode through an external wire. A common galvanic cell is the Daniell cell, shown below. The Zn (s) gives up its electrons Socratic
11.1: Galvanic Cells – Chemistry LibreTexts
In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a Chemistry LibreTexts
17.7: The Direction of Electron Flow and its Implications
In an electrolytic cell, the cathode is the electrically negative electrode. The direction of current flow in any cell can be reversed by the application of a sufficiently Chemistry LibreTexts
Lecture 2: Basic Physics of Galvanic Cells & Electrochemical
The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the spontaneous redox reactions taking place MIT OpenCourseWare
17.2 Galvanic Cells – Chemistry Fundamentals – University of
By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the Cu Cu foil) and the cathode is the electrode where reduction occurs University of Central Florida Pressbooks
Galvanic (voltaic) cells (video) | Khan Academy
Galvanic (or voltaic) cells use a thermodynamically favored redox reaction to generate an electric current. Each half-reaction takes place in a separate compartment, or half-cell, containing an electrode. The electrode where oxidation occurs is the anode, and Khan Academy
Galvanic Cells (Voltaic Cell) – Definition, Working
In a galvanic cell, when an electrode is exposed to the electrolyte at the electrode-electrolyte interface, the atoms of the metal electrode have a tendency to generate ions in the electrolyte solution leaving behind the BYJU’S
17.2 Galvanic Cells – Chemistry 112- Chapters 12-17
Galvanic or voltaic cells involve spontaneous electrochemical reactions in which the half-reactions are separated ( Figure 2) so that current can flow through an external wire. The Pennsylvania State University
Voltaic Cell | How Does It Work?
Introduction To Galvanic Cells \U0026 Voltaic Cells
9.2 Describe How Current Is Conducted In An Electrolytic Cell [Sl Ib Chemistry]
Galvanic Cells Explained -In Under 5 Minutes.
Galvanic Cells (Voltaic Cells)
Galvanic Cell.Swf
Electron Flow Vs Conventional Current. | How Do 1000 Million Electrons Flow Inside Wire?
In Which Direction Do Electrons Flow In A Galvanic Cell, From Anode To Cathode Or Vice Versa?
Link to this article: where do electrons flow in a galvanic cell.
See more articles in the same category here: https://bmxracingthailand.com/what
